Chapter 8
1. What are the two principal types of energy?
2. Classify the following according to the type of energy.
a. You, standing on the tope of a ski slope with your snowboard on.
b. You, snowboarding down the mountain.
c. You, wearing a parachute and peering out of an airplane.
d. You, plummeting to Earth just before your parachute opens.
e. The pizza and beverage that waits for you at the bottom of the mountain.
3. For each of the following processes, predict whether it is exothermic or endothermic.
a. CO2(s) ---> CO2(g)
b. CO2(g) ---> CO2(s)
c. O2(g) ---> 2 O(g)
d. 2 O(g) ---> O2(g)
4. Assume that you place two beakers of water out at room temperature (about 20oC). Beaker A contains 50 grams of water at 80oC. Beaker B contains 50 grams of water at 15oC.
a. What will happen to the temperature of the water in each beaker?
b. For which beaker will this change be complete sooner?
c. For one of the beakers, the change is endothermic. For the other beaker, the change
is exothermic. Which beaker is which?
5. For each of the following processes, does entropy increase or decrease?
a. Melting ice
b. Burning paper
c. Stirring sugar into your coffee
d. Breaking an egg
6. Describe how a combustion engine makes a car move?
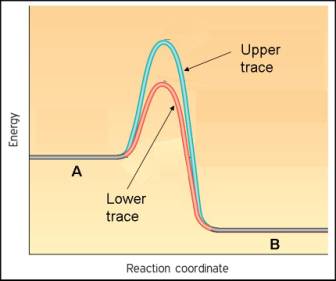
7. The energy diagram for the reaction A ---> B is shown here under two different conditions, upper and lower traces.
a. Which reaction is faster, the upper or the lower pathway?
b. For the upper pathway, which of the following must be true?
Releases energy
Exothermic
Increasing entropy
Relatively fast
Spontaneous
c. What is the difference between the upper and lower traces if what is shown is the same reaction?
8. Why is dissolving ionic solids an endothermic process? Where does the energy go?
9. Green plants use the photosynthesis reaction to make sugar from carbon dioxide and water:
6CO2(g) + 6H2O(g) ---> C6H12O6(s) + 6O2(g)
a. Would you predict that this is an exothermic or endothermic reaction?
b. Would you predict that this reaction has a positive (increased disorder) or negative entropy change?
c. What is the role of sunlight in this reaction?