
Proteins: Globular and Membrane
Globular Proteins:
Soluble.
Most enzymes are globular.
The primary way globular proteins maintain their tertiary structure is called the Hydrophobic Effect.
Charged, polar hydrophilic residues on the surface.
Non-polar residues seclude themselves in a hydrophobic "core" area.
Other ways of maintaining 30 structure "Salt Bridges" and "Disulfide Bridges"

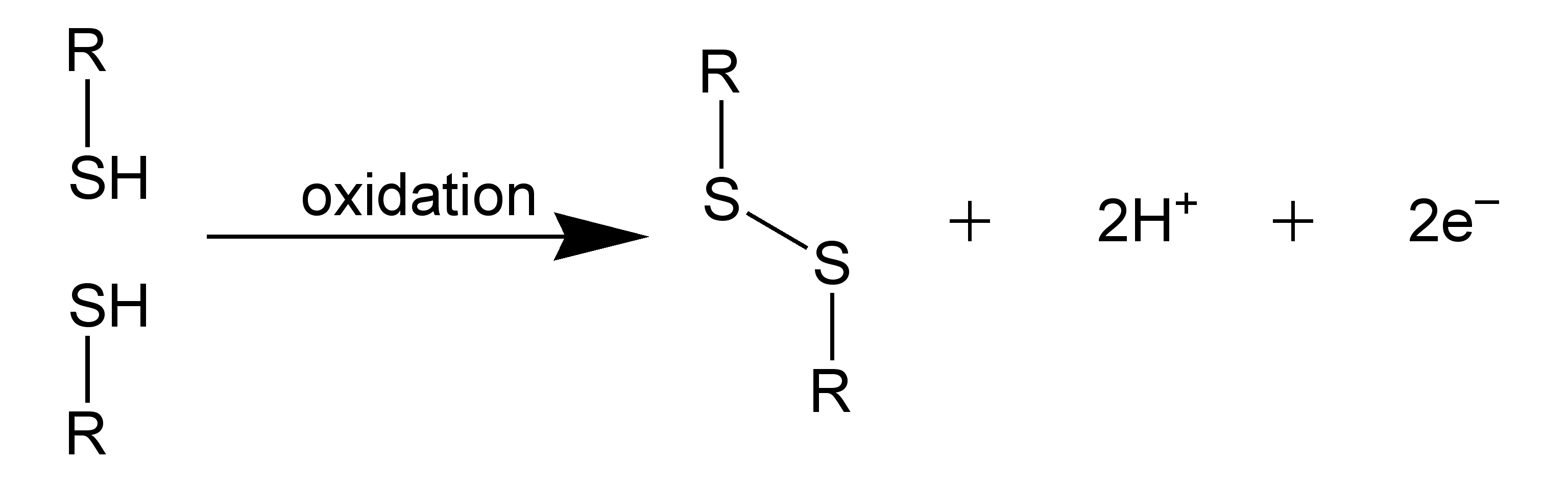
One feature that most globular proteins share is the ability to bind certain small molecules.
The site where binding occurs is the active site.
The small molecules that bind are called substrates.
The most common type of globular protein that is capable of binding a small molecule is an enzyme.
Enzymes:
Are Globular Proteins.
Are catalytically active proteins which convert substrate to product.
Are specific for their substrate and reaction.
Often require a supplementary chemical group or
CO-FACTORS-
Most enzymes require a supplementary chemical group, called a co-factor,to be fully active. There are organic and inorganic co-factors, many enzymes contain both.Oraganic: there are two types, co-enzymes and prosthetic groups.
| Co-enzyme | Prosthetic Group |
| Soluble, easily removed | Covalently Attached |
Inorganic:
Fe, Cu, Mg
Glycoproteins:

>Globular proteins, especially those in circulation, are often modified to increase solubility and enhance biological activity.
>One method is by the covalent attachment of a sugar unit, glycosylation ------> glycoprotein
>The variety of sugars, sugar modifications and different linkages give glycoproteins a very high information content.
Enzymatic Glycosylation
>enzyme directed, specific
>generally involves "anhydride" formation
Non-Enzymatic Glycosylation
>non-specific
> "Schiff" base formation
Membrane Proteins:
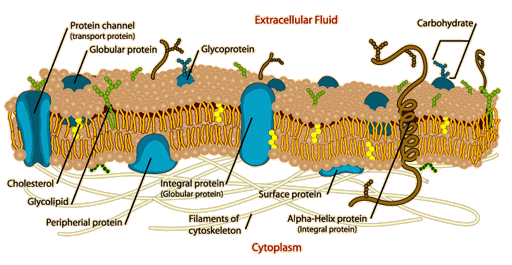
>often contain a globular domain
> are anchored to a membrane
>can be categorized as:
Receptors-extracellular globular domains can bind specific molecules such as, hormones.
Transporters-span the membrane, allow passage of molecules across the membrane.
Cell-Cell Recognition Proteins-usually glycoproteins, high information content allows very specific interactions between cells.
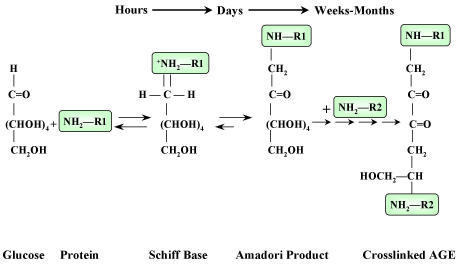
Clinical Correlate: Non-Enzymatic Glycosylation
>In diabetic patients, blood sugar can reach very high levels ------> the aldehyde groups on these glucose molecules can spontaneously react with free amines on circulating proteins.
>Since Hb is an abundant blood protein, it is frequently glycosylated in a "non-enzymatic" fashion.
>Short-term ("schiff" base formation) non-enzymatic glycosylation is reversible.
>If glucose levels remain uncontrolled for months (long-term), the attached glucose will form a non-reversible cross-link with a second amino group.
>These compounds are called Advanced Glycosylation Endproducts (AGE's).
>AGE's can be measured and are a good indicator of long term blood glucose control in the diabetic patient.
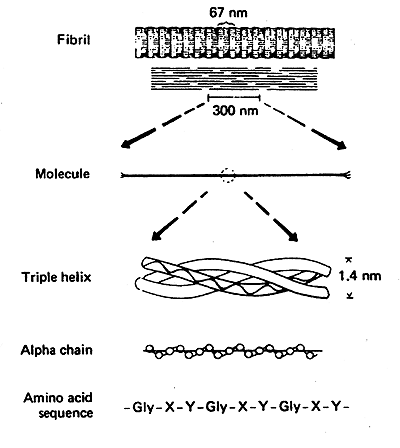
The three main types of structural or fibrous protiens fround in the human body are: collagens, elastins and keratins.
Collagen:
~25% of the proteins found in the body are classified as collagens.
structural element of connective tissue
unique type of triple helix, stabilized by crosslinks
Amino Acid Sequence: Glycine at every third position.
-Gly-X-Y-Gly-X-Y-Gly-X-Y
Collagen Helix: Only 3.0 residues per turn vs. 3.6 for an average a-helix.
Triple Helix: Three collagen helices wound very tightly together.
Collagen Fibrils: The triple helices are arranged in staggered fashion (covalent crosslinks vital to the structure of mature collagen).
Important specializations seen in collagen:
1) Proline is very abundant and can be hydroxylated by prolyl hydroxylase to form hydroxyproline. Co-factor for prolyl hydroxylase is: ascorbic acid (vitamin C).
Proline --------> Hydroxyproline
2) Lysine is abundant and can be hydroxylated by lysyl hydroxylase to form hydroxylysine. Co-factor for lysyl hydroxylase is: ascorbic acid (vitamin C).
Lysine --------> Hydroxylysine
3) Hydroxylysines can be glycosylated on their newly acquired -OH groups. (sugar attachment)
HO-Lysine ------> "Glycosylation"
4) Cysteine residues at the ends of the collagen helices are oxidized to form disulfide bridges, this places the chains in proper orientation for triple helix formation.
2 Cysteine Residues -------> Disulfide Bridge
5) Covalent crosslinks are formed between allysine and lysine during fibril formation:
(a) Oxidation of certain lysine and hydroxylysine residues converts them from amines to active aldehydes (allysines). The enzyme for this reaction is lysyl oxidase, co-factors: copper, Vitamin B6.
Lysine-NH3+ ------> Allysine-C=O
(b) A reaction between these aldehydes and free amino groups on other lysine residues forms a "Schiff" base, then covalent crosslink.
Lysine-NH3+ + Allysine-C=O ---------> "Schiff" Base
Collagen Maturation:
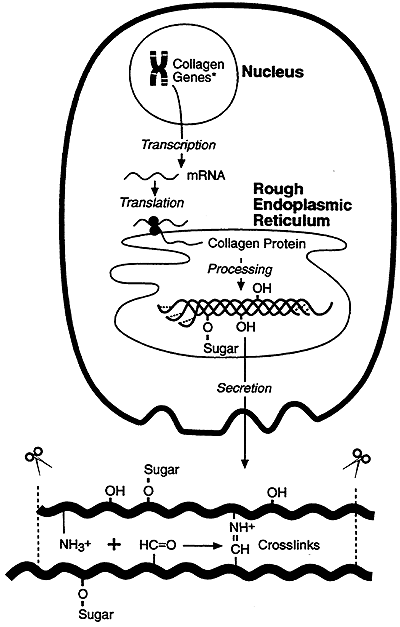
Nucleus:
Transcription
ER:
HO-Pro and HO-Lys
Glycosylation some HO-Lys
Disulfide Formation
Folding "Triple Helix"
Extracellular:
Assembly into Fibrils
Lys, HO-Lys to Allysine (active Aldehydes)
"Schiff" Base Formation (Covalent Crosslinks)
Elastin: is another important class of insoluble fibrous proteins.
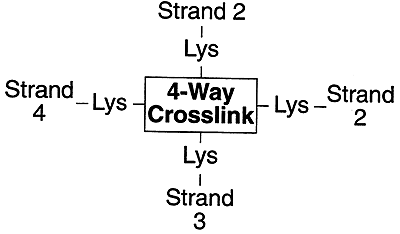
Elastin lacks the genetic complexity, diversity and helical structure of collagen.
Two features elastin and collagen share:
1) Some prolines are hydroxylated. (not Lysine, so no glycosylation)
2) Some Lysines are oxidized to active aldehydes (allysine).
Unique to elastin:
Four lysines are crosslinked in a unique fashion called desmosine.
Keratins: insoluble structural proteins found in skin, nails and hair
There are two types of keratins:
a-Keratins ( a-helix)
b-Keratins ( b-sheet)
In hair and nails the a-helices of adjacent strands are crosslinked by disulfide bridges.
Nails: there are numerous disulfide bridges making them resistant to chemical alteration ----> "hard"
Hair: there are relatively few disulfide bonds and they can be easily disrupted with "reducing" agents.
Permanent Wave:
1) Disulfides are reduced
2) Cosmetic rearrangement (curlers)
3) Free -SH groups are oxidized to reform disulfides
Some heritable diseases related to collagen:
Antibodies:
-circulating glycoproteins synthesized by white blood cells (lymphocytes)
-crucial part of the body's defense system or "Immune Response"
All antibodies belong to the family of proteins called immunoglobulins, named for their role in the immune response, i.e. their ability to specifically recognize antigens (foreign invaders).
Antibody Structure:
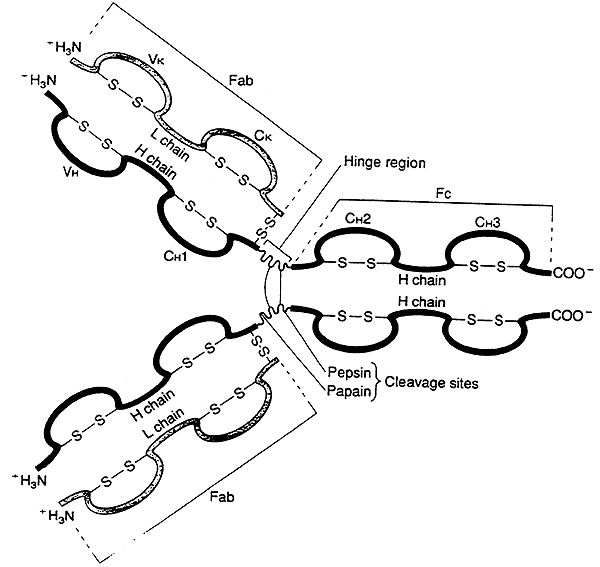
-Immunoglobulins share a common structure of three subunits arranged in a trimeric unit.
-Although, some immunoglobulins are more complex and contain multiples of this unit.
Features of the Antibody-
1) Two identical heavy chains (H).
2) Two identical light chains (L).
3) The chains are joined by interchain disulfide bonds.
4) The subunit is often depicted as a "Y".
IgG Molecule (an illustration of the basic immunoglobulin unit):
(a) several globular domains, intersecting at a central "hinge" region
(b) each globular domain is stabilized by intrachain disulfide bonds
(c) the variable (V) regions for both the heavy (H) and light (L) chains always occur together in a globular domain at the upper tips of the "Y"
(d) the two variable regions on each side form the binding site for an antigen, the immunoglobulin molecule has two identical binding sites
(e) "the two binding sites are identical within a single Ab molecule, no two Ab's are alike"
(f) the body is capable of producing millions of different variable regions
(g) the constant (C) regions within a given class of immunoglobulins remains relatively constant
"Hinge" region:
-susceptible to digestion by proteases, i.e. pepsin or papain
-enzymes cleave the immunoglobulin into two fragments:
Fab ("antigen binding") fragment contains the variable regions -----> binding sites
Fc ("crystallizable") fragment mediates many of the functions common to an immunoglobulin class.
(Fc portion is derived solely from the heavy chains, differences in the heavy chain constant regions are what distinguish one class of immunoglobulin from another.)
Antigen Recognition:
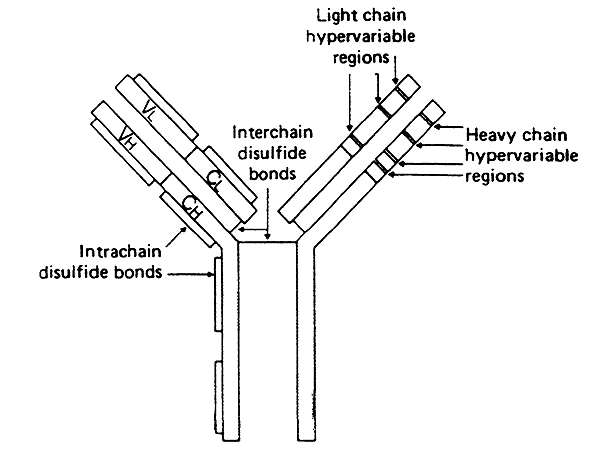
-a single antigen binding site is formed by the convergence of the variable regions from one heavy chain and one light chain (two binding sites per subunit).
-not all residues w/in the variable region change from one immunoglobulin to the next
-some do not vary much, forming the three-dimensional framework of the globular domain
-w/in each light-chain variable region are three hypervariable regions
-w/in each heavy-chain variable region are four hypervariable regions
Hypervariable Regions:
>vary tremendously forming the complementarity-determining regions (CDR's)
>in the heavy-chain variable regions there are four CDR's
>in the light-chain variable regions there are three CDR's
>the combination of seven CDR's in one binding pocket help create the vast repertoire of binding sites that form an important part of the immune system
Hapten:
>each CDR faces towards the antigen binding pocket and recognizes, but does not elicit antibody response, a small set of features on the antigen surface
>set of features is the hapten and may represent a specific chemical grouping
Types of Interactions Important in Antigen Binding:
Ionic Bonds
Salt Bridges
Hydrophobic Interactions
Hydrogen Bonding
Clinical Correlate:
Vaccines
Inject the surface component of a "dead" virus or bacteria (one that has been modified so it is non- virulent).
Ab's will be produced for this surface component.
If the vaccinated individual is later exposed to a virulent form of the virus his/her immune system will be prepared -------> making short work of the virus.
Success: chicken pox
More Difficult: HIV, has the ability to alter it's cell surface proteins in endless permutations
Clinical Correlate: Autoimmune Diseases
Body no longer recognizes "self", develops Ab's toward it's own cells.
© Dr. Noel Sturm 2021