Proteins-Fibrous (Structural)
The three main types of structural or fibrous protiens fround in the human body are: collagens, elastins and keratins.
Collagen:
~25% of the proteins found in the body are classified as collagens.
structural element of connective tissue
unique type of triple helix, stabilized by crosslinks
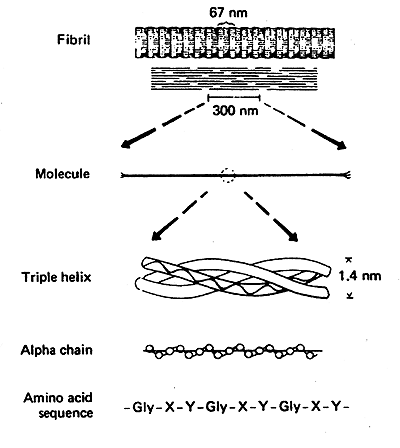
Amino Acid Sequence: Glycine at every third position.
-Gly-X-Y-Gly-X-Y-Gly-X-Y
Collagen Helix: Only 3.0 residues per turn vs. 3.6 for an average a-helix.
Triple Helix: Three collagen helices wound very tightly together.
Collagen Fibrils: The triple helices are arranged in staggered fashion (covalent crosslinks vital to the structure of mature collagen).

Important specializations seen in collagen:
1) Proline is very abundant and can be hydroxylated by prolyl hydroxylase to form hydroxyproline. Co-factor for prolyl hydroxylase is: ascorbic acid (vitamin C).
Proline --------> Hydroxyproline
2) Lysine is abundant and can be hydroxylated by lysyl hydroxylase to form hydroxylysine. Co-factor for lysyl hydroxylase is: ascorbic acid (vitamin C).
Lysine --------> Hydroxylysine
3) Hydroxylysines can be glycosylated on their newly acquired -OH groups. (sugar attachment)
HO-Lysine ------> "Glycosylation"
4) Cysteine residues at the ends of the collagen helices are oxidized to form disulfide bridges, this places the chains in proper orientation for triple helix formation.
2 Cysteine Residues -------> Disulfide Bridge
5) Covalent crosslinks are formed between allysine and lysine during fibril formation:
(a) Oxidation of certain lysine and hydroxylysine residues converts them from amines to active aldehydes (allysines). The enzyme for this reaction is lysyl oxidase, co-factors: copper, Vitamin B6.
Lysine-NH3+ ------> Allysine-C=O
(b) A reaction between these aldehydes and free amino groups on other lysine residues forms a "Schiff" base, then covalent crosslink.
Lysine-NH3+ + Allysine-C=O ---------> "Schiff" Base
Collagen Maturation:
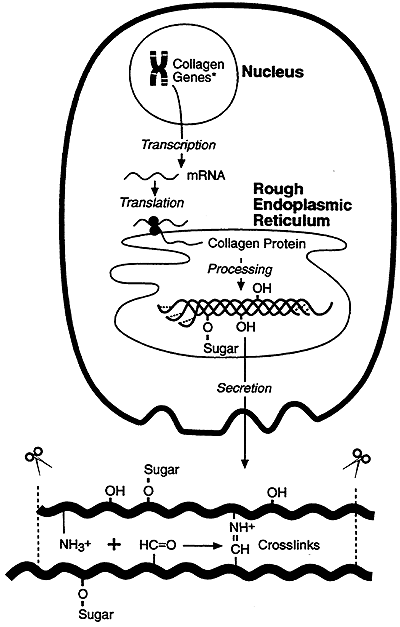
Nucleus:
Transcription
ER:
HO-Pro and HO-Lys
Glycosylation some HO-Lys
Disulfide Formation
Folding "Triple Helix"
Extracellular:
Assembly into Fibrils
Lys, HO-Lys to Allysine (active Aldehydes)
"Schiff" Base Formation (Covalent Crosslinks)
Elastin: is another important class of insoluble fibrous proteins.
Elastin lacks the genetic complexity, diversity and helical structure of collagen.
Two features elastin and collagen share:
1) Some prolines are hydroxylated. (not Lysine, so no glycosylation)
2) Some Lysines are oxidized to active aldehydes (allysine).
Unique to elastin:
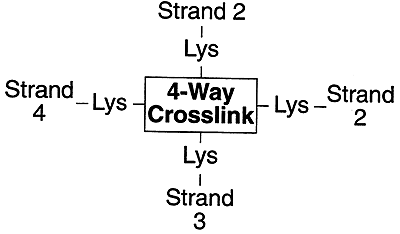
Four lysines are crosslinked in a unique fashion called desmosine.
Keratins: insoluble structural proteins found in skin, nails and hair
There are two types of keratins:
a-Keratins ( a-helix)
b-Keratins ( b-sheet)
In hair and nails the a-helices of adjacent strands are crosslinked by disulfide bridges.
Nails: there are numerous disulfide bridges making them resistant to chemical alteration ----> "hard"
Hair: there are relatively few disulfide bonds and they can be easily disrupted with "reducing" agents.
Permanent Wave:
1) Disulfides are reduced
2) Cosmetic rearrangement (curlers)
3) Free -SH groups are oxidized to reform disulfides
Some heritable diseases related to collagen:
© Dr. Noel Sturm 2020