Myoglobin/Hemoglobin
Myoglobin (Mb):
single chain, high a-helical content, globular
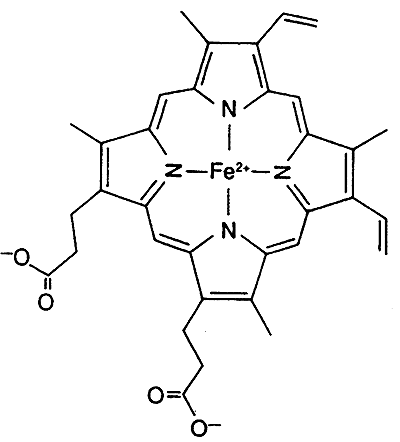
Both Myoglobin and hemoglobin (Hb)contain the Heme Prosthetic Group

Inorganic Co-factor: iron, Fe2+ (ferrous), the binding of O2 keeps Fe2+ from oxidizing to Fe3+
Myoglobin and hemoglobin contain both organic and inorganic co-factors
The six coordination sites in the Heme Prosthetic Group:
four sites are occupied by nitrogen (relatively the same plane)
fifth site by nitrogen from the proximal histidine residue
sixth site by oxygen or carbon monoxide (nitrogen from distal histidine)
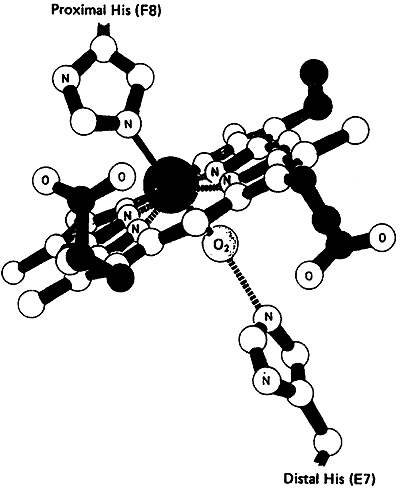
Carbon monoxide is a toxic by-product of metabolism and heme actually has a higher affinity for CO then O2.
Mb and Hb have developed a mechanism (as yet unknown) to discriminate against binding by CO, still, CO binds better than O2 and a measurable amount of Mb and Hb in the body exist bound to CO.
Myoglobin
Normal O2 Pressures:
| Location | Approximate pO2 (mmHg) | Comments |
| Air | 150 | Normal atmospheric pressure at sea level |
| Lung Capillaries | 100 | Where direct gas exchange occurs |
| Venous Blood | 40 | Where gas exchange to tissues occurs |
| Muscle | 20 | Under normal activity |
| O2-Deprived Muscle | >5 | Under conditions of strenuous exercise |
Myoglobin binds oxygen when the pO2is high and releases oxygen at very low pO2
Oxygen Affinity Curve for Myoglobin:
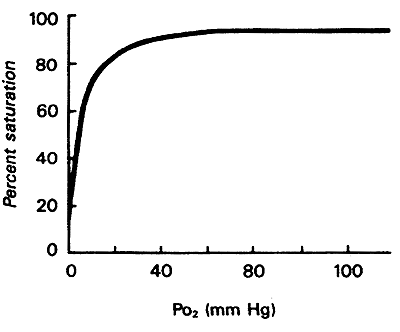
When all Mb molecules have O2 bound-100%
When no Mb molecules have O2 bound-0%
Looking at curve:
1. high affinity for O2, at most physiological conditions O2 remains bound to Mb.
2. only when O2 falls very low, exercise etc., Mb releases O2 to other tissues
3. "the role of Mb is to store O2 for release during crisis involving O2 deprivation"
i.e. seals and whales contain abundant Mb (deep dives)
Hemoglobin (Hb): has a structure very similar to Mb
with one very notable exception, Hb is a tetramer with two types of subunits, alpha and beta.
two a and two b (a2b2, referred to as HbA-normal hemoglobin)
This means that hemoglobin has quaternary structure and must have all four subunits, each with it's own individual primary, secondary, tertiary structure, to be a fully functioning protein.
Cooperativity/Cooperative Binding:
When an O2 molecule binds to the first site a conformational change occurs in the tetrameric structure which affects binding to the other subunits.
Oxygen binding curve is a characteristic sigmoidal ("S") shape.
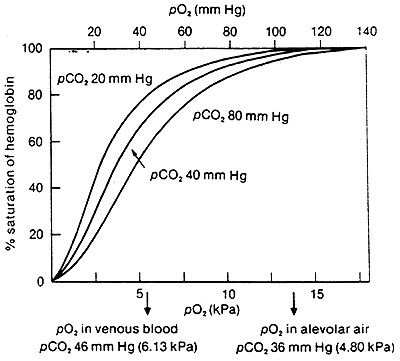
Comparison of Oxygen Affinity Curve for Hemoglobin vs. Myoglobin:
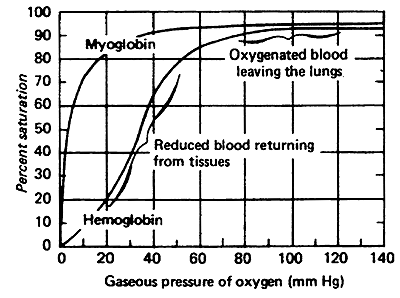
Hb takes up O2 in the lungs and releases it to interior tissues
At pO2of 100 mmHg, Hb is saturated
At pO2 of 20 mmHg, Hb loses most of its O2
Even at 40 mmHg Hb is loosing some of it's O2
Hemoglobin is actually a dimer of dipeptides.
When oxygen binds we have Oxyhemoglobin (R)- one pair of the dipeptides rotates, opening a 150 cleft
Deoxyhemoglobin (T)- two pairs sit in close contact (as in the pisture above)
The story of the Salt Bridges:
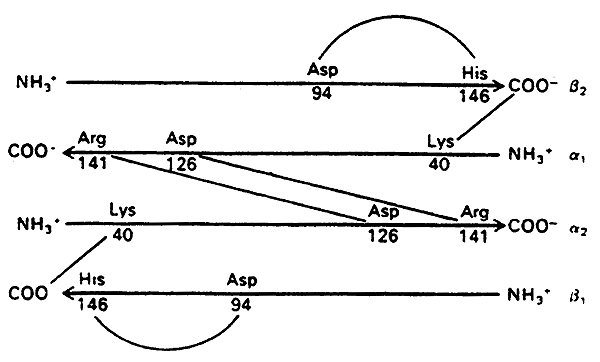
DeoxyHb (T state)- stabilized by salt bridges between the chains
When oxygen binds OxyHb (R state)- the salt bridges are disrupted
Hence Oxygen binding induces a conversion from the T to the R state, increasing affinity for itself (cooperativity).
Cooperativity:
Binding one molecule of O2 enhances binding the subsequent O2 molecules.
When one O2 binds it provokes a T to R conversion on the other subunits.
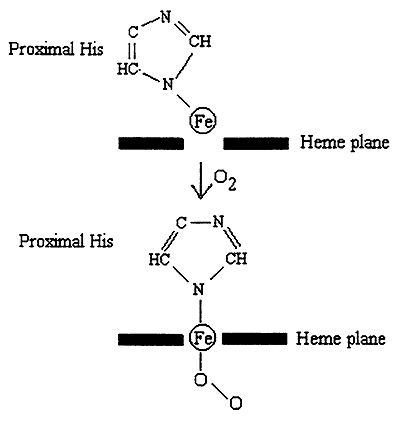
1. Bound O2 "pulls" the Iron atom into the plane of the heme.
2. Movement of the Iron pulls the proximal His.
3. Movement of His affects the structure ---> disrupts salt bridges.
4. This "frees up" another subunit, which now exists in an R-like state with increased affinity for O2.
5. Binding of O2 to the second subunit triggers the same sequence, enhancing binding to the third subunit etc.....
Protons (H+), CO2, 2,3-bisphosphoglycerate (BPG) (in tissues): induce a conversion from the R to the T state, decreasing affinity for oxygen and causing it to be released to the tissues.
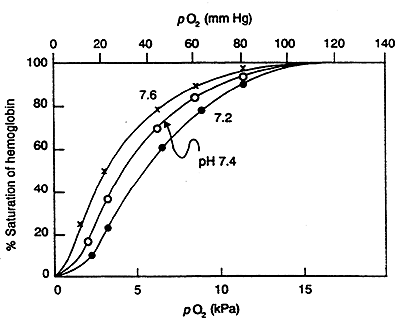
Bohr (Proton) Effect:
O2 binding to Hb is sensitive to physiological pH changes.
i.e. Contracting Muscle- lactic acid is produced, hence the H+ concentration increases, pH decreases -------->promotes O2 dissociation (fulfills greater requirement for O2).
H+ antagonizes the binding of O2 to Hb.
In lungs, higher pH increases affinity of Hb for O2 (reverse of what is happening in muscle).
In terms of R and T:
H+ induces an R to T conversion, His becomes protonated giving it a (+) charge, induces salt bridge formation with Asp a characteristic of the T state.
Carbon Dioxide:
CO2 is a byproduct of metabolism.
Accumulation of CO2 is a characteristic of active metabolism and increased O2 requirements by the tissue.
CO2 lowers the affinity of Hb for O2 so that in the tissues O2 is released.
In lungs CO2 is kept lower by expiration increasing the affinity of Hb for O2 (reverse of what is happening in tissues).
In terms of R and T:
CO2 binds to the N-terminus of Hb, HbNH-COO-, this forms a salt bridge stabilizing the T state.
2,3-Bisphosphoglycerate:
BPG is produced in the RBC from the metabolism of glucose
BPG lowers the affinity of Hb for O2
Accumulation of BPG is a characteristic of active metabolism and increased O2 requirements by the tissue.
In lungs, when O2 is bound, the cavity where BPG binds becomes to small, thus the increased affinity for O2 in the lungs.
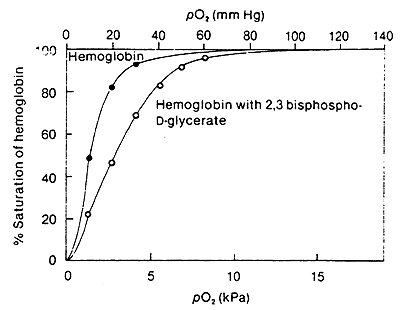
In terms of R and T:
The (-) BPG interacts with (+) charged residues (Lys, His etc.) cross-linking the b- subunits.
Again these interactions stabilize the T state.
Clinical Correlate:
Fetal Hemoglobin
a2g2 tetramer instead of a2b2
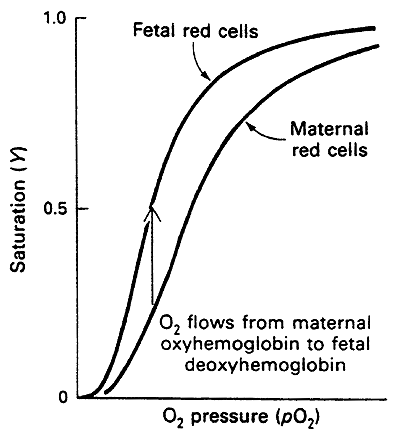
In the gamma (g) subunit much of the His becomes Ser
The lack of His disrupts salt bridge formation
Hence HbF is more relaxed and has a higher affinity for O2 than adult Hb
This higher affinity optimizes the transfer of O2 from maternal to fetal circulation thus oxygenating the fetus
Fetal and normal Adult Hb are Isoforms, distinct forms of a protein that have the same function but are found in different tissues, i.e. a2g2 vs. a2b2
Note:
© Dr. Noel Sturm 2021-The amount of Hb in the blood is so high that it contributes to the buffering capacity of blood.
-However, the bicarbonate system is still the major blood buffer.
-Hb does have the ability to accept or donate protons through its His residues (36 His residues per tetramer, so this cannot be discounted....).