Enzymes: Catalysis and Kinetics
General Properties of Enzymes:
1) Enzymes are biological catalysts they speed up reactions with-out being consumed.
2) Enzymes are highly specific for their substrates.
3) Enzymes display a high degree of reaction specificity which discourages wasteful byproducts.
4) Co-factors: organic coenzymes, and prosthetic groups (covalent) or inorganic (non-covalent)
5) In a metabolic pathway one reaction or one enzyme always represents the rate-limiting step, this determines the rate for the entire pathway.

Types of Enzyme Reactions:
Transferases-catalyze the transfer of groups from one molecule to another.
Hydrolases-cleave a substrate using water (hydrolysis).
Oxidoreductase-involved in oxidation-reduction, transfer of e-'s between molecules.
Lyase-catalyzes the lysis of a substrate w/out water or oxygen.
Ligases-catalyze the joining of two substrates, often by the elimination of water.
Isomerases-catalyze the rearrangement of a single substrate.
Energy of Enzyme Reactions:
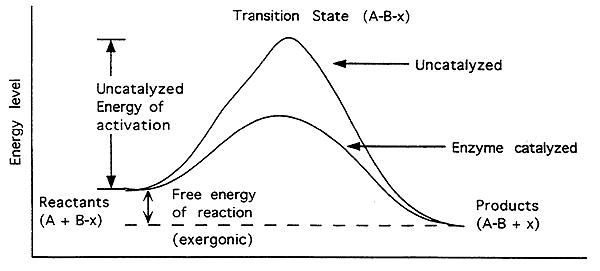
Free Energy of an Enzyme Catalyzed Exergonic Reaction-
Exergonic (Exothermic):
Energy level of reactants higher than products.
The free energy change (DG) is negative (-).
Reaction will proceed spontaneously.
Free Energy of an Enzyme-Catalyzed Endergonic Reaction-
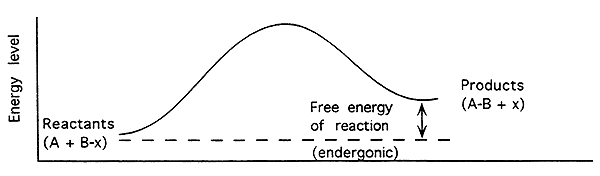
Endergonic (Endothermic):
Energy level of products higher than reactants.
The free energy change (DG) is positive (+).
Reaction will not proceed spontaneously.
Temperature:
Raising the temperature of a system will increase the energy of the interacting molecules and the frequency with which they collide.
Normally the body operated within a very narrow temperature range so this has little effect on enzyme rates.
However, when a fever develops the rate of all metabolic pathways will increase ------->raising the energy demands in the body, hence the phrase "feeding a fever".
Enzyme Kinetics:
Michaelis-Menten Equation-
V = Vmax [S] / Km + [S]
V = velocity (rate of reaction)
Vmax = when all of the enzyme molecules contain bound substrate (saturated), maximal velocity
[S] = concentration of the substrate
Km = concentration of the substrate needed to give half maximal velocity (1/2 Vmax), a measure of affinity, enzyme for substrate
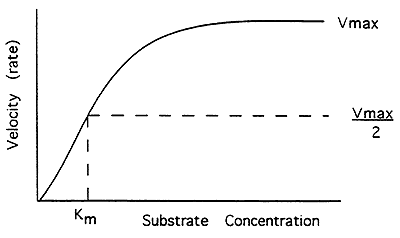
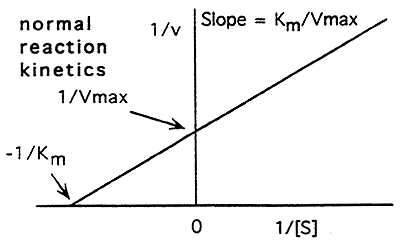
The Michaelis-Menten Equation Can be Re-Arranged:
V = Vmax [S] / Km + [S]
Invert:
1 / V = Km + [S] / Vmax [S]
Re-Arrange:
1 / V = Km / Vmax [S] + [S] / Vmax [S]
1 / V = Km / Vmax . 1 / [S] + 1 / Vmax
(Y = m x + b), thus we have the equation of a line and can now look at enzyme kinetics graphically using the Lineweaver-Burke plot
Lineweaver-Burke Plot:
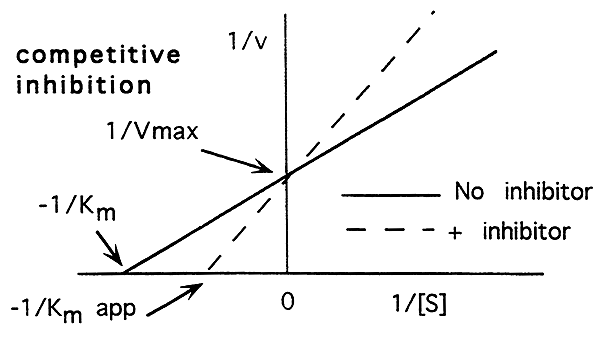
Enzyme catalyzed reactions can be inhibited. There are two types of inhibitors, competitive and non-competitive.
Competitive Inhibition:
-A competitive inhibitor binds to the active site of the enzyme.
-A competitive inhibitor does not change the maximum rate for the reaction.
-A competitive inhibitor lowers the enzymes affinity for its substrate.
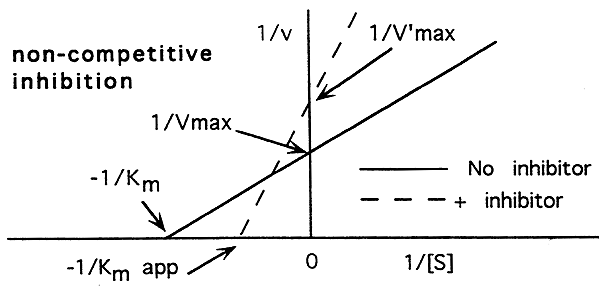
Non-Competitive Inhibition:
-A non-competitive inhibitor binds to a site other than the active site.
-A non-competitive inhibitor slows the maximum attainable rate of the reaction.
-A non-competitive inhibitor slightly decreases an enzymes affinity for its substrate by altering the shape of the active site.
© Dr. Noel Sturm 2020