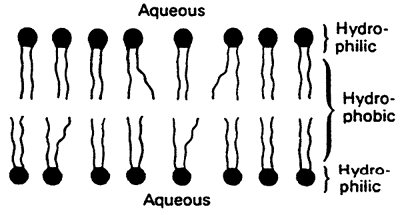
Membranes I and II
Function:
Membranes are external boundaries for cells and separate the compartments of the cell.
Common Features:
1. Membranes are sheetlike, just a few molecules thick and form closed boundaries between cell compartments.
2. Membranes contain lipids and proteins, with small amounts of CHO's linked to lipids and proteins.
3. Lipids in membranes are small with hydrophobic and hydrophilic portions. Lipid bilayers provide a barrier to the diffusion of polar molecules.
4. Characteristic functions of membranes are mediated by specific proteins, serving as pumps, channels, receptors, energy transducers and enzymes.
5. Most membrane components associate through noncovalent interactions.
6. Membranes are asymmetrical, with two sides of the membrane differing from each other.
7. Lipid and protein molecules often diffuse rapidly in the plane of the membrane.
Bilayer Formation:
Hydrophobic interactions provide the primary driving force for the formation of bilayers.
Simple Bilayer:

Saturated fatty acid chains pack easily and have a higher melting temperature (Tm). Butter is a solid at room temperature so has a high Tm.
Unsaturated fatty acids have a lower Tm. Canola oil is a liquid at room temperature so has a low Tm.
Cholesterol impedes motion of the hydrocarbon tails making membranes less fluid.
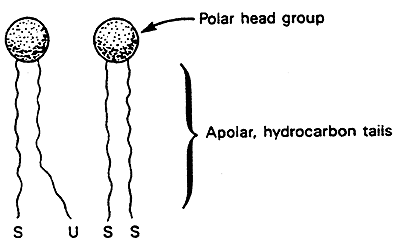
The degree of saturation of the "tails" affects the stability of the membrane. Saturated fats pack more easily than unsaturated fats. A high percentage of unsaturated fats lowers the temperature at which a membrane will become rigid.
Fluid Mosaic Model :
A nice picture of a membrane.
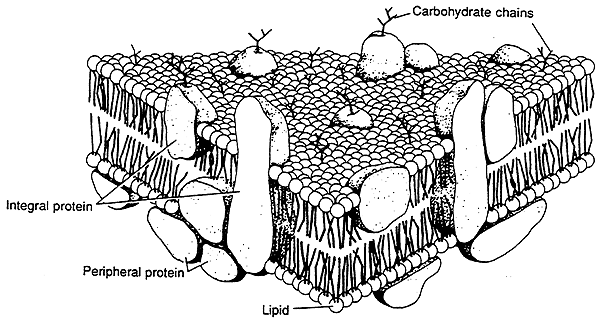
Membrane Carbohydrates:
1. Common monosaccharides associated with membranes are: glucose, galactose, fructose and mannose.
2. Polysaccharides attached to membranes and are built from monosaccharides attached to each other via glycosidic bonds.
3. Hexoses can be linked from any of 5 positions on the molecule making possible numerous different structures and giving the membrane "high information content".
4. Both membrane proteins and lipids can be glycosylated.
5. In glycoproteins, the sugar residues are attached to nitrogen of Asn (N-linked) or via hydroxyl of a Ser or Thr (O-linked).
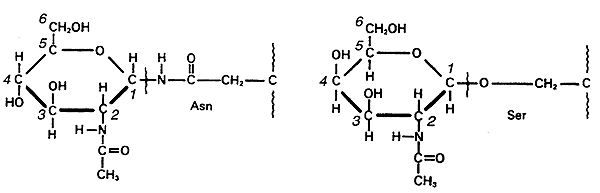
6. Both glycoproteins and glycolipids are important in cell-cell recognition.
Clinical Correlate: Blood Group Antigens (Blood Typing)
1. The ABO blood group antigens are distinct polysachharides found mainly on glycoproteins on the RBC membrane.
2. The polysachharides are constructed in the ER and Golgi apparatus by glycosyl transferases.
3. Individuals express different glycosyl transferases.
4. Thus, each blood type is characterized by a different polysachharide structure on the surface of the RBC.
5. The different polysachharide chains are characterized (classified) as A, B, O or AB.
Membrane Asymmetry:
1. Membrane components are asymmetrically distributed across the bilayer.
2. Membranes are asymmetrically oriented:
pumps drive transport in one direction
receptors bind molecules on the outside
3. CHO's are asymmetric because they are found on the outside of the surface of the cell.
Membrane Lipids:
Sphingolipids and Glycolipids:
Sphingomyelin (an important spingolipid)-
A lipid found in brain, blood cells and lung surfactant.
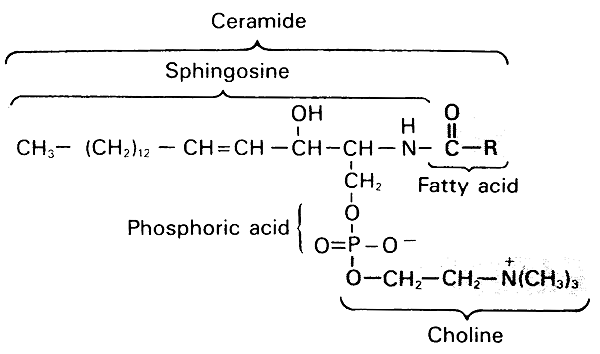
Shingomyelin
Glycolipids:
Have an identical structure to shingolipids, except the phosphoryl choline headgroup is replaced with a monosachharide (sugar).
Membrane Proteins:
Peripheral Membrane Proteins:
-Are either extracellular or intracellular.
-Though associated with membranes they can be easily removed.
Extracellular Proteins:
Protein ligands specific for cell surface receptors.
Proteins of the extracellular matrix.
Intracellular Proteins:
Attach to lipid anchors or to integral proteins.
These proteins can be modified covalently by lipids ----> localizes them to specific membranes.
Many of these modified proteins are oncoproteins --> cause cancer if they become malfunctional.
Integral Membrane Proteins:
Span the bilayer and thus have both intra- and extracellular domains.
The transmembrane domain is always a-helical.
They can not be removed from the membrane without very harsh treatments.
Integral membrane proteins fall into three classes: antigens, receptors and translocators.
1. Antigens:
Integral membrane proteins that are recognized by antibodies.
See antibody/antigen lecture for review.
2. Receptors:
Are required for the specific action of hormones, transmitters and growth factors.
Two general classes of membranes receptors are: growth factor receptor tyrosine kinases (RTKs) and small molecule 7 transmembrane helix receptors.
(a) RTKs bind hormones such as insulin
They contain:
-an extracellular ligand binding domain
-a single a-helical transmembrane domain
-an intracellular domain which can phosphorylate tyrosine residues on proteins.
(b) Small Molecule 7 Transmembrane Helix Receptors
-bind hormones such as epinephrine and glucagon
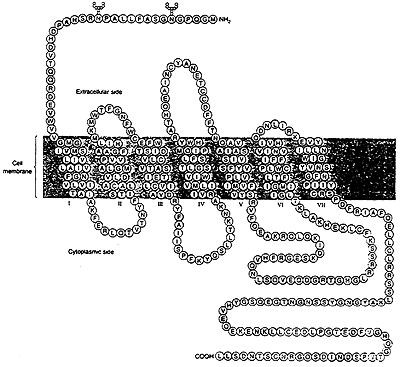
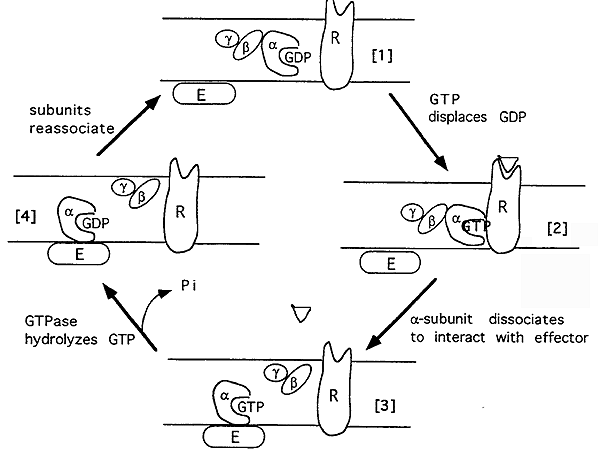
Most small molecule 7 transmembrane receptors are G-Proteins- activate other membrane proteins which bind GTP.
G-Proteins
3. Transporters (Translocators):
The major function of membranes is that of a permeability barrier allowing the cell to maintain distinctly different environments between compartments.
Cells have evolved transport mechanisms to move things across membranes.
Transporters may be active or passive.
Passive Transport (channel- or carrier-mediated) molecules are transported with the Electrochemical (concentration) Gradient
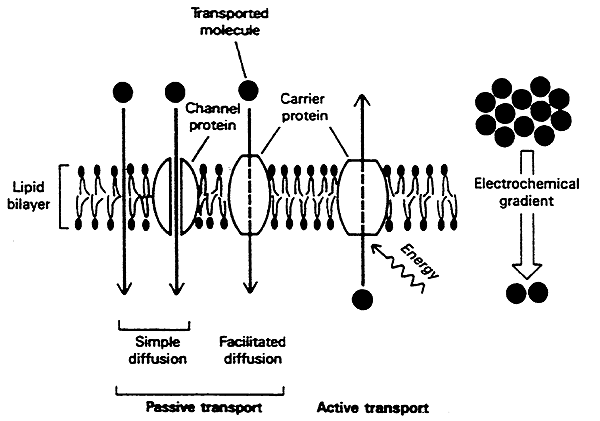
Active Transport:
In some cases the cell "pumps" molecules against its concentration (electrochemical) gradient.
This is active transport because energy must be expended.
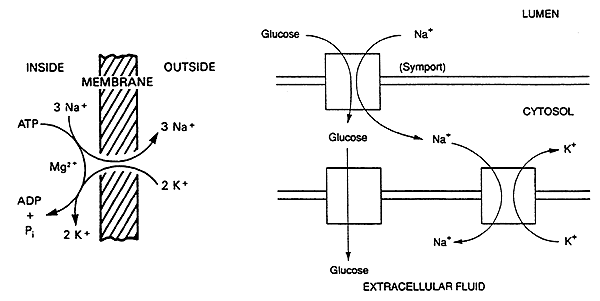
Na+ / K+ ATPase (or Na+ Pump)
This "pump" is found in the plasma membrane of ALL mammalian cells.
Its function is to pump three Na+ ions out of the cell and two K+ ions into the cell, at the expense of one ATP molecule.
© Dr. Noel Sturm 2011