_and_Carl_Ferdinand_Cori.jpg)
Introduction
Biochemistry is the study of the molecules and chemical reactions of life.
Major players:
Friedrich Wohler- synthesized urea, an organic compound, by heating an inorganic compound, ammonium nitrate. Showed for the first time that an organic ("living") compound can be synthesized from an inorganic ("non-living") compound.
Eduard Buchner- identification of enzymes as the catalysts of biological reactions. Showed that extracts of yeast cells could catalyze the fermentation of sugar to alcohol and carbon dioxide.
Emil Fischer- during catalysis an enzyme and its substrate (reactant) combine to form an intermediate compound. Only a molecule with suitable structure can serve as a substrate for a given enzyme....specificity.
_and_Carl_Ferdinand_Cori.jpg)
Gerty Cori and Carl Cori- jointly won the Nobel Prize in 1947 for their discovery of the Cori cycle
Chemistry of Life
Elements found in the human body:
| ELEMENT |
Abundance in Body(% of dry weight) |
Biomolecular Examples |
|
Carbon (C) Oxygen (O) Hydrogen (H) |
50.0 20.0 10.0 |
Proteins, Carbohydrates, DNA/RNA, Lipids, Heme |
| Nitrogen (N) | 8.0 | Protein, DNA/RNA, Heme |
| Calcium (Ca) | 4.0 | Bone |
| Phosphorus (P) | 2.5 | Bone, Lipids, DNA/RNA |
| Potassium (K) | 1.0 | Electrolyte |
| Sulfur (S) | 0.8 | Proteins |
| Sodium (Na) | 0.4 | Electrolyte |
| Chloride (Cl-) | 0.4 | Electrolyte |
| Magnesium (Mg) | 0.1 | DNA/RNA, Proteins |
| Iron (Fe) | 0.01 | Proteins, Heme |
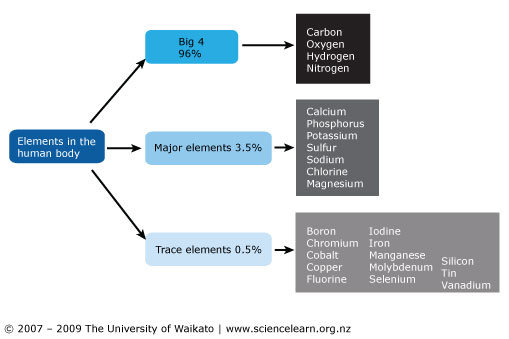
The four most abundant and important are Carbon (C), Oxygen (O), Hydrogen (H) and Nitrogen (N)
They comprise the majority of atoms in the FOUR classes of molecules found in the human body:
1. proteins
2. carbohydrates
3. nucleic acids (DNA and RNA)
4. lipids (including fat)
| Class of Molecule | Composition | Role in Human Body |
| Lipids | C, H, O (N, P) | Membrane Structure, Fuel, Fuel Storage |
| Carbohydrates (CHO's) | C, H, O (N) | Fuel, Fuel Storage, Cell-Cell Recognition |
| Proteins | C, H, O, N (S) | Enzymes, Tissue Structure, Fuel |
| Nucleic Acids (DNA, RNA) | C, H, O, N, P | Genetic Information Storage and Transfer |
Also important...
Phosphorus-nucleic acids
Calcium, Phosphorus-skeleton
Sodium, Potassium, Chloride-body fluids (electrolytes)
Magnesium-DNA and RNA
Iron-heme(participates in binding oxygen)
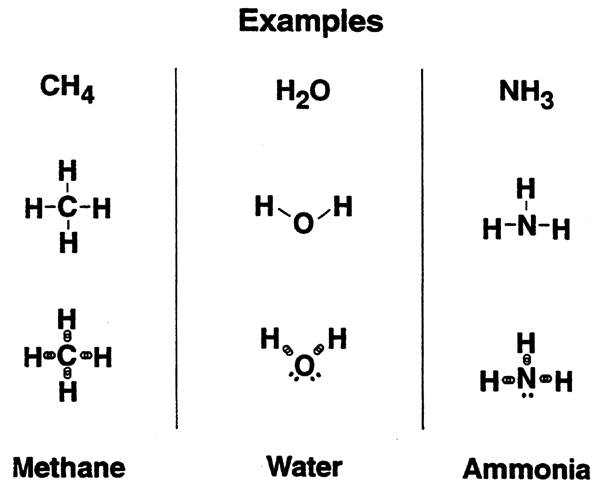
Covalent Bonds: A Review....
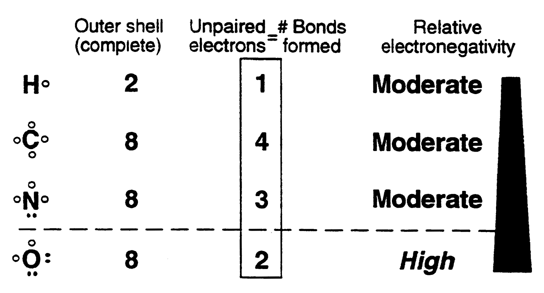
Covalent bonds are the main structural framework of proteins, carbohydrates, lipids, nucleic acids.
Oxygen has a high attraction for electrons to complete its outer shell.
i.e. oxygen is more elecronegative than than hydrogen. Result: oxygen will posses a partial negative charge while hydrogen becomes partially positive.---POLAR
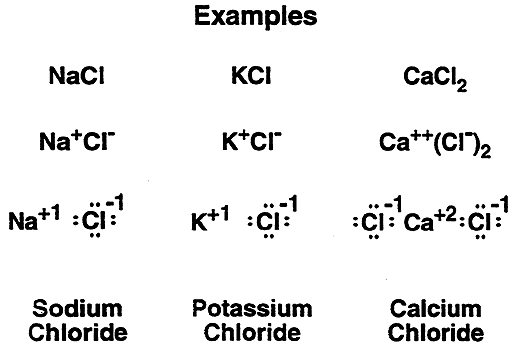
Ionic Bonds: A review.....
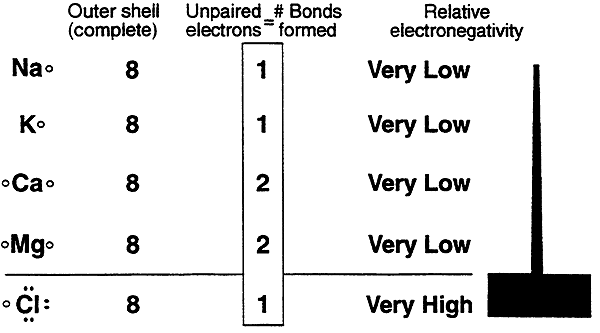
Extreme differences in electronegativity between elements leads to ionic bonds.
Ions can actually drift apart especially in a polar solvent such as water.
In biological systems where water is the solvent Na and Cl nearly always exist as separate ions.
Water:

Most important biological molecule.
Important feature: Hydrogen Bonding Ability
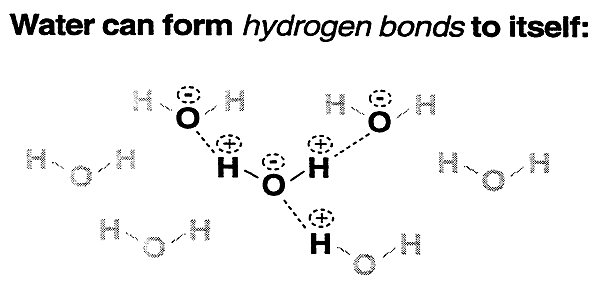
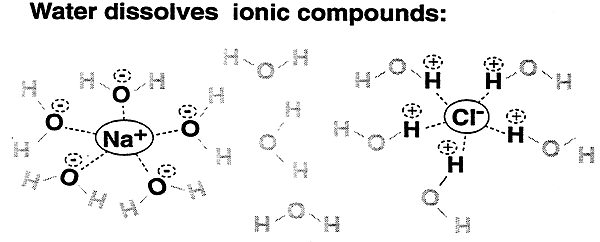
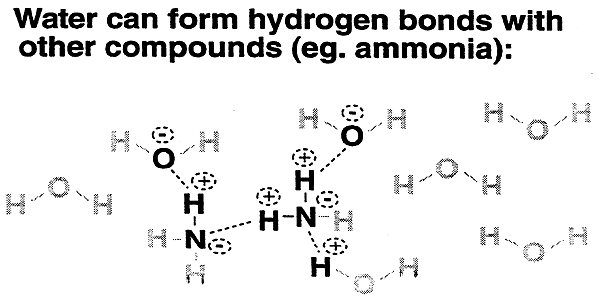
The ability of water to hydrogen bond:
1. gives water great cohesion and resistance to vaporization.
2. allows for solvation of biomolecules.
Hydrophobic:
"water fearing", i.e. long-chain hydrocarbon
Hydrophilic:
"water loving", i.e. ionic, polar compounds
Amphiphilic:
both "water fearing" and "water loving"
Molecules which contain both a "hydrophilic" and "hydrophobic" region, i.e.
![]()
"A Fatty Acid"
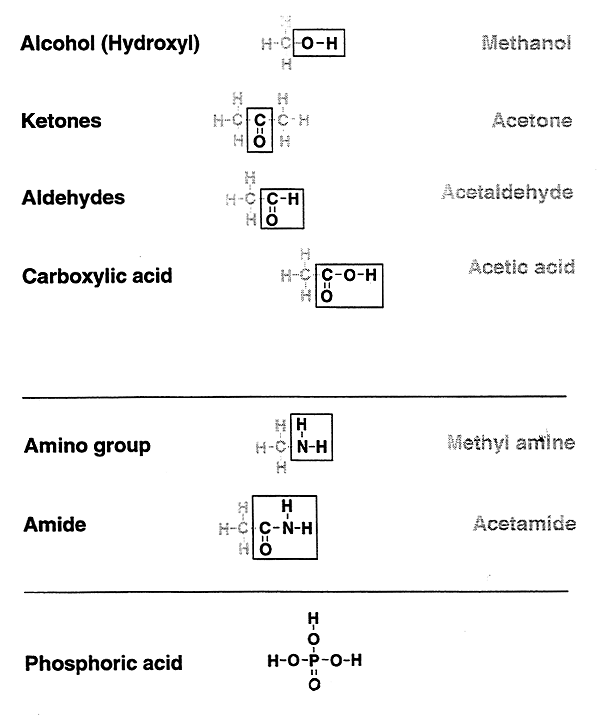
Review of functional groups important in the study of biochemistry:
The Cell
The basic unit of life...
Every organism is composed of either a single cell or many cells.
There are two major classes of cells:
Eukaryotic- complex internal structure, including a prominent nucleus.
Prokaryotic- are less complex and much smaller.
Prokaryotic Cells:
Usually single celled organisms, such as bacteria.
E. coli-
1. lacks a nucleus, a large DNA molecule packed in the nucleoid region.
2. Cell-wall-made of a network of covalently linked carbohydrate and peptide chains.
Eukaryotic Cells:
Include plants, animals, fungi.
The Nucleus: Control center of the cell, containing 95% of its DNA.
DNA replication and transcription of DNA into RNA.
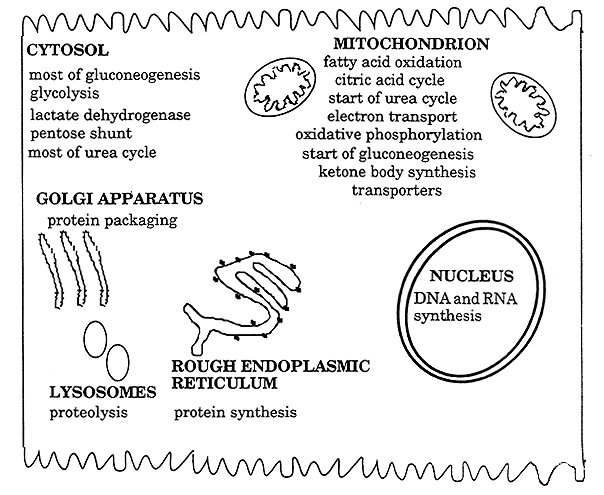
Endoplasmic Reticulum (ER):
Aqueous region enclosed w/in the ER is the lumen.
Protein synthesis/export.
Many enzyme systems involved in the metabolism of lipids.
Golgi Apparatus: Protein modification/sorting/packaging/transport.
Mitochondria: Central role in energy transduction.
Oxidative energy metabolism.
Chloroplasts: Sites of photosynthesis in plants and algae.
Mitochondrial Matrix: The inner mitochondrial membrane and the matrix contain many of the enzymes involved in aerobic energy metabolism.
Sites of Biochemical Processes in the Cell:
© Dr. Noel Sturm 2020