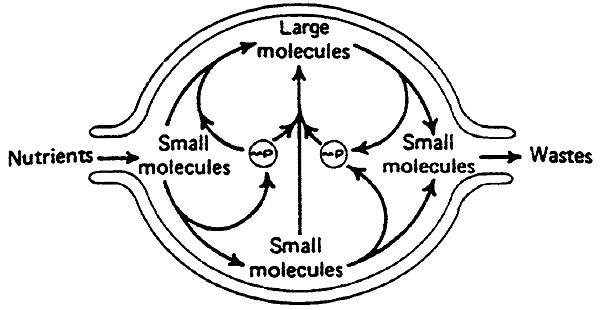
Introduction to Metabolism
Some hints for studying the metabolic pathways:
Organize, understand, condense, memorize
Minimize the amount of material that you memorize.
Use diagrams that have meaning for you.
Sort out trivia.
How to Study Metabolism:
Develop an "Information Sorter"
1. Purpose of the metabolic pathway-what is the overall function?
2. Names of molecules entering (substrates) and leaving (products) the pathway.
3. How the pathway interacts with other pathways.
4. General metabolic conditions under which the pathway is stimulated or inhibited.
5. Identify control points-which steps of the pathway are regulated?
6. Identify regulatory molecules and the direction that they push the metabolic pathway.
7. Names of molecules in the pathway and how they are connected.
8. Names of reactants and products for each regulated enzyme.
9. Names of individual reactants and products for all enzymes in a pathway.
10. Names of reactants and products for each enzyme making or using ATP, NADH, GTP, FADH2.
11. Specific molecules that inhibit or activate specific enzymes.
12. Essential vitamins and co-factors involved in the pathway.
13. Consequences of an enzyme deficiency in a pathway.
Metabolic Terms:
1. Anabolism- synthetic process.
2. Catabolism- degradative process.
3. Committed Step- the first reaction unique to a pathway.
4. Rate-Limiting Step- slowest reaction of the pathway, determines the rate of the pathway.
5. Steady-State- unidirectional flow of metabolites ---> concentration of metabolites that remains relatively constant until the system is perturbed.

Nine types of Metabolic (Enzymatic) Regulation:
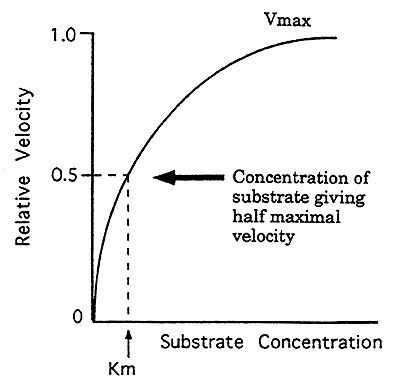
1. Enzyme Kinetics: most common type of regulation (Michaelis-Menten)
When the substrate concentration is AT or BELOW the Km then the reaction rate is very sensitive to small changes in substrate concentration.
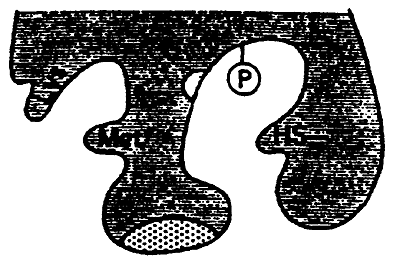
2. Allosterism: when a compound acts at a site on the enzyme other than the active site. Can be stimulatory or inhibitory.
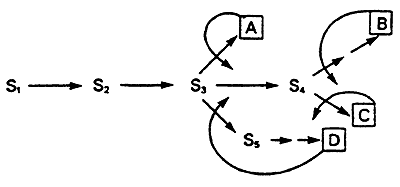
3. Feedback Inhibition: negative modulation by an end-product.
4. Covalent Modification: covalent bonding of some chemical group to the enzyme. Can be stimulatory or inhibitory. i.e. phosphorylation.
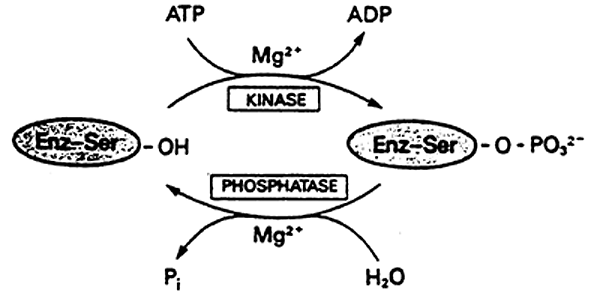
5. Polymerization: association or dissociation of subunits of the complex.
6. Regulatory Proteins: stimulate or inhibit enzymes through direct interaction.
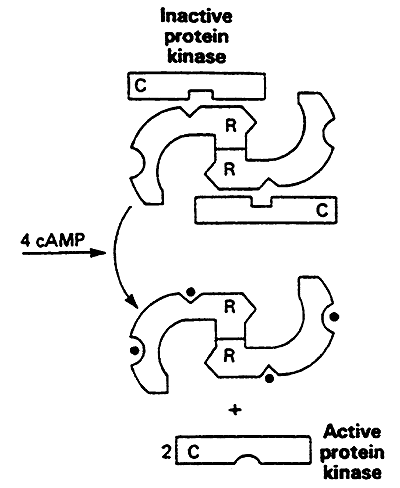
7. Proteolytic Activation: removal of a peptide segment from the protein molecule.
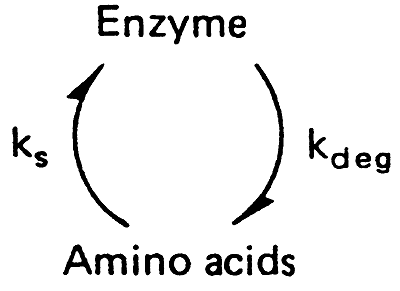
8. Induction: synthesis of new enzyme by initiation of transcription of the gene.
Repression: the opposite of this process(inhibition of synthesis)
The overall balance here represents turnover.
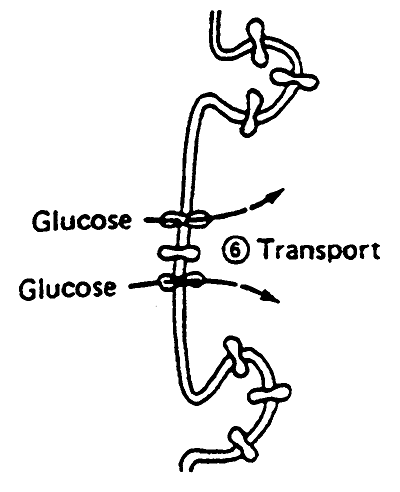
9. Translocation: across plasma and intracellular membranes.
Systems of Energy metabolism:
Overview of the Metabolic Pathways:
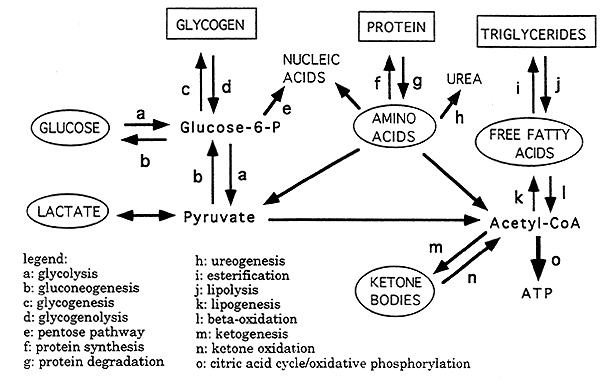
Three Energy Storage Forms (squares):
glycogen (carbohydrates)
protein (amino acids)
triglycerides (fatty acids)
Five Circulating Fuels (circles):
glucose
lactate
amino acids
free fatty acids
ketone bodies
Key Metabolic Intermediates ("crossroads" in metabolism):
glucose-6-phosphate
pyruvate
acetyl CoA
Most Energy in the Body is Produced by the complete oxidation of Acetyl CoA to ATP (the Citric Acid Cycle combined with Electron Transport).
Thermodynamics in Metabolism
Energy is required for many processes including locomotion and biosynthesis
In mammalian cells energy is produced primarily via oxidative metabolism, the Electron Transport Chain. The Electron Transport Chain converts NADH and FADH2, generated in the Citric Acid Cycle, into ATP via oxidative phosphorylation.
Exceptions:
anaerobic metabolism in RBC's (no mitochondria)
skeletal muscle undergoing high intensity exercise
plants energy is derived from photosynthesis
High Energy Phosphate Bonds:
ATP is the most common carrier of free energy
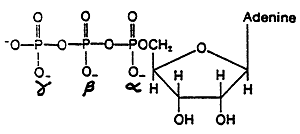
The energy of ATP is stored in two phosphoanhydride bonds-
between the g and b phosphates, -7.3 kcal/mol
between the b and a phosphates, -6.6 kcal/mol
removal of the a phosphate does not release energy
Other High Energy Phosphate Compounds:
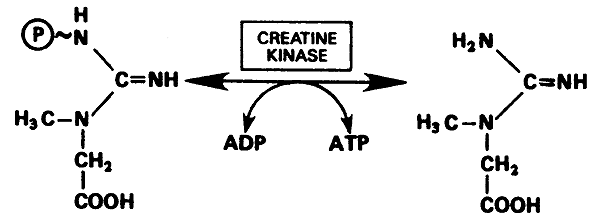
phosphocreatine, -10.3 kcal/mol
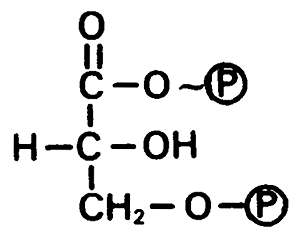
1,3-bisphosphoglycerate, -11.8 kcal/mol (glycolysis)
phosphoenolpyruvate, -14.8 kcal/mol (glycolysis)
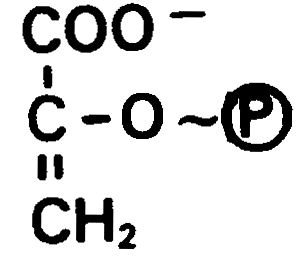
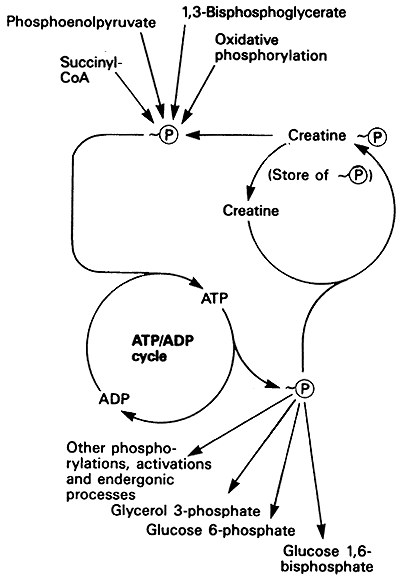
High Energy Phosphate Transfer Potential:
The release of phosphate from ATP and other high energy phosphate compounds drives endergonic reactions.
Two Types of Phosphorylation
Substrate-level Phosphorylation:
Substrate level phosphorylation involves phosphate transfer during enzymatic reactions of metabolic pathways (i.e. glycolysis).
Oxidative Phosphorylation:
ATP is synthesized using the energy produced by the reduction of oxygen to water in mitochondria, also known as the "Electron Transport Chain". Since reductive processes require electrons, electron carriers (NADH and FADH2) are essential in energy metabolism.
Review of Electron Carriers in Metabolism:
Reductants (donate electrons): NADH, NADPH, FADH2
Oxidants (accept electrons): NAD+, NADP+, FAD
Free Energy:
-the portion of the total energy of a system that is available for useful work
The change in free energy for an enzyme reaction in the cell is denoted DG
DG = DG0' + 2.3RT log [(products) / (reactants)]
DG0' = standard free energy constant (pH=7.0)
-DG = exergonic reaction, spontaneous
+DG = endergonic reaction, not spontaneous
remember, a +DG0' can still lead to a -DG
also, an endergonic reaction can be driven by being coupled to an exergonic reaction, DG for a metabolic pathway is the sum of the free energy values for each enzyme reaction in the pathway. For a pathway to proceed the overall DG needs to be negative.
LaChatlier Principle: decrease in product concentration or an increase in reactant concentration lowers DG ---> reaction becomes more favorable.
Equilibrium: forward and backward reaction rates are the same, no net flux in either direction
DG = 0
DG0' = -2.3 RT log Keq
Keq = [products] / [reactants]
Adenylate Kinase: an "Important Metabolic Regulator"
ATP + AMP <---> 2 ADP
During intense exercise: large conversion of ATP to ADP with the muscle attempting to phosphorylate ADP back to ATP, this increase in ADP pushes the reaction to the left (mass action effect)
i.e. a 20% decrease in ATP leads to an almost 3-fold increase in ADP and ultimately a 1,400% increase in AMP
The increase in AMP is what's important here......
Because AMP is an allosteric regulator that signals a low energy state in cells --------->
rise in AMP increases metabolism through pathways to restore energy (i.e. glycolysis, citric acid cycle etc.).
© Dr. Noel Sturm 2020