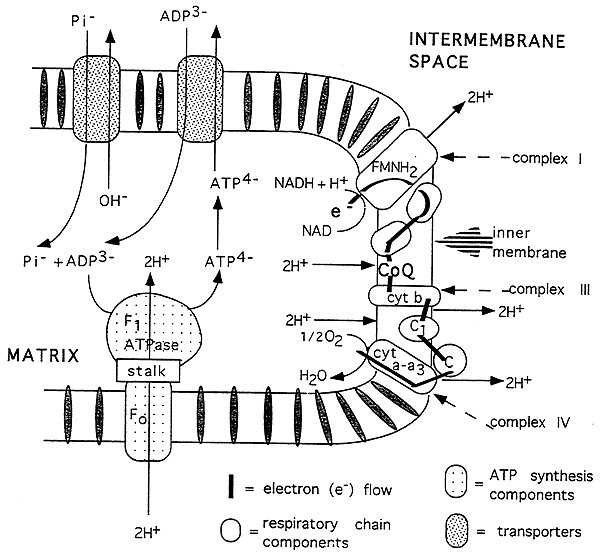
Electron Transport Test
Respiratory Chain, "Oxidative Phosphorylation"
Purpose of the Pathway: convert NADH and FADH2 into ATP
The principle part of the chain consists of three complexes (I, III, IV) which are integral proteins of the inner mitochondrial membrane (not important to RBC's...) and interact via mobile carriers of electrons.
A fourth complex (II) contains succinate DH.
The components have progressively more positive E0 values leading to electron transfer and energy production for pumping protons out of the matrix.
The components span the inner membrane asymmetrically to facilitate proton pumping.
Note: Because electrons passing through complex II bypass complex I, FADH2 yields one fewer ATP molecule than NADH, which feeds electrons through complex I.
Inhibitors of Electron Transport:
1. Rotenone: inhibits complex I, rat poison and insecticide.
2. Antimycin A: an antibiotic which blocks complex III.
3. Cyanide: inhibits terminal electron transfer to oxygen, Complex IV.
4. Carbon Monoxide: inhibits cytochrome oxidase by competing with an oxygen- binding site, Complex IV.
Asymmetrical Orientation of Electron Transport Chain:

The ATP synthase complex, ADP and phosphate translocases.
Protons are pumped out of the matrix at complexes I, III and IV (2 per complex).
The resulting proton gradient is used to drive ATP synthesis: 2 H+ per ATP produced.
The proton gradient also creates a charge difference across the inner mitochon. membrane.
(membrane potential: more negative on the inside)
ATP Synthase Complex:
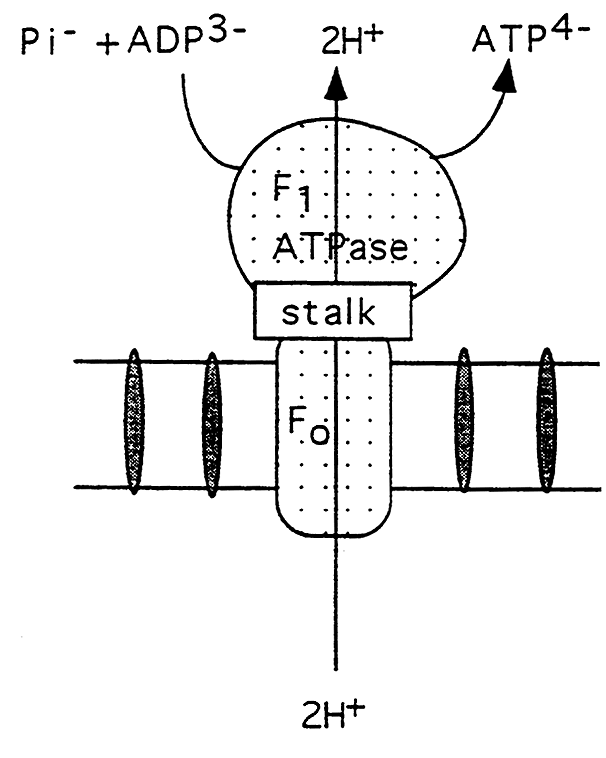
(a) F1 (ATPase) is the sperical protein extending into the matrix.
(b) Contains 5 different subunits and the catalytic site for ATP synthesis.
(c) the complex contains sites that change in their affinity for ATP as protons flow through the complex.
(d) This proton flow allows the ATPase to reverse direction and synthesize ATP.
(e) F0 is the integral oligomer attached to F1 via a stalk.
(f) The F0 contains the proton channel.
(g) The stalk regulates proton flow and ATP synthesis, contains an F1 inhibitor and is the oligomycin sensitive region.
Uncoupler, Dinitrophenol (DNP): binds protons, is hydrophobic and can dissipate a pH gradient by equilibrating H+ (protons).
i.e. Dinitrophenol (DNP), causes ATP formation to cease but oxygen consumption remains rapid in an attempt by the mitochondria to maintain the proton gradient.
Energy is released as heat and body temperature rises.
Rapid O2 consumption in uncoupling is due to loss of respiratory control.
Oligomycin:
Oligomycin is an antibiotic which binds to the stalk preventing proton flow to the ATPase.
Respiration will diminish because the proton gradient can no longer be utilized.
Respiration will slow even in the presence of large amounts of ADP and phosphate.
(-) citric acid cycle (because of accumulation of NADH)
(-) PDH (increased amounts of NADH and Acetyl CoA)
P / O Ratio:
The P / O (phosphate:oxygen) ratio represents the amount of Pi used (ATP synthesized) per atom of oxygen consumed.
1. Electron flow from NADH to O2 pumps protons ar three sites to yield 3 ATP (P / O = 3)
2. Succinate (via FADH2) bypasses site 1 giving 2 ATP (P / O = 2)
3. Uncoupling: P / O = 0, oxygen is consumed with no ATP production.
© Dr. Noel Sturm 2020