Adrenal Steroid Hormone Biosynthesis
General Structural Features for the Steroid Hormones:
Intact four ring system (except vitamin D where the B-ring
has been opened).
Often contain hydroxyl side chains ----> sterols.
Hydroxyl groups are denoted b
if they are oriented above the plane (solid line).
".......................................... "a
if they are oriented below the plane (dashed line).
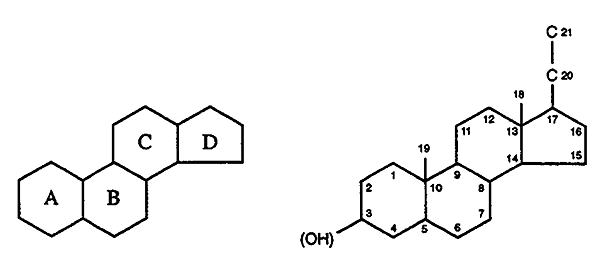
General Structure and
Numbering Scheme
Steroid hormones are classified according to their physiologic
action and tissue of origin:

Biosynthesis of the Adrenal Steroid Hormones:
The adrenal steroid hormones are synthesized from cholesterol derived mostly from the plasma (some cholesterol is synthesized in situ from acetyl-CoA).
Much of the cholesterol is esterified (by ACAT) and stored in lipid droplets.
Upon stimulation of the adrenal zona fasciculata
(ZF) by ACTH (adrenocorticotropic hormone) (cAMP second messenger), or the adrenal
zona glomerulosa (ZG) by angiotensin II (IP3
/ Ca2+
and DAG) ---------> an
esterase is activated and the free cholesterol formed is transported into the
mitochondria.
In the mitochondria a cyctochrome P-450 cleavage enzyme (P-450scc or 20,22-lyase) converts cholesterol to pregnenolone.
Cholesterol Side-Chain Cleavage (SCC): A "Key"
Reaction in Adrenal Steroid Hormone Synthesis
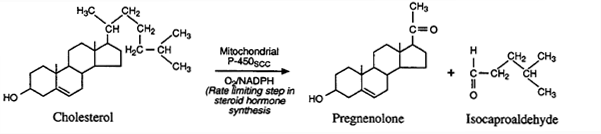
All mammalian steroid hormones are formed from cholesterol
via pregnenolone.
Subcellular Compartmentalization of Glucocorticoid
Biosynthesis:
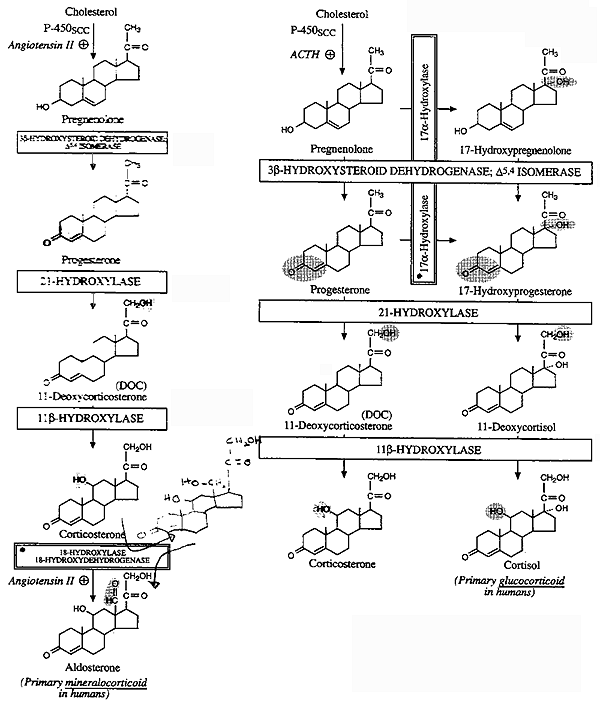
Corisol (glucocorticoid) synthesis occurs in the ZF
Aldosterone (mineralocorticoid) synthesis occurs in the ZG
Key to Figure, "Biosynthesis of Mineralo- and Gluco-
Corticoids"
i) Pregnenolone is converted to progesterone by
3b-hydroxysteroid DH
(3b-OHSD) and D5-D4
isomerase.
ii) Progesterone is hydroxylated at C21
to form 11-deoxycorticosterone (DOC). DOC is an active (Na+-retaining)
mineralocorticoid.
iii) Next hydroxylation is at C11
producing corticosterone.
iv) 18-Hydroxylase acts on corticosterone to form
18-hydroxycorticosterone.
v) The alcohol is oxidized to an aldehyde forming
aldosterone. This conversion is regulated by renin-angiotensin and K+.
Concentration of receptors is "up-regulated" by high K+
and "down-regulated" by low K+.
Angiotensin II stimulates the conversion of cholesterol to pregnenolone,
and corticosterone to 18-hydroxycorticosterone and aldosterone via Gp
induced increases in IP3/Ca2+
and DAG (more next lecture...).
i) 17a-Hydroxylase acts upon either progesterone or pregnenolone to form 17a- hydroxyprogesterone and/or 17a-hydroxypregnenolone.
ii) 17a-hydroxyprogesterone is hydroxylated at C21 to form 11-deoxycortisol which is then hydroxylated at C11 to form cortisol. Cortisol is the most potent natural glucocorticoid hormone is humans.
Adrenal steroid biosynthesis involves the shuttling of precursors
between mitochondria and the ER.
StAR: Steroidogenic Acute Regulatory Protein
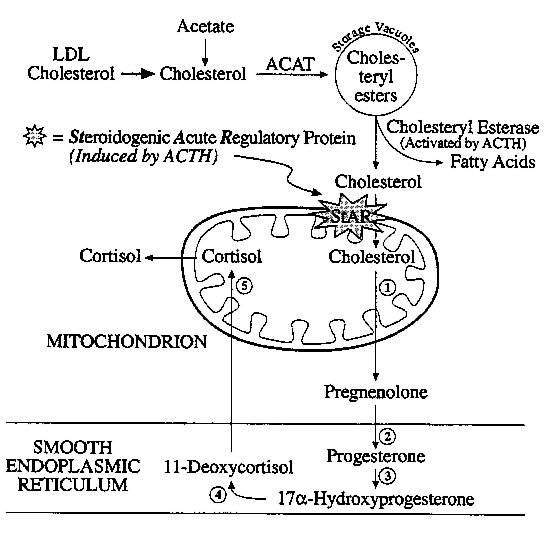
(1) P-450scc
/ 20,22-lyase (Mitochondria)
(2) 3b-hydroxysteroid
DH and D5,4
isomerase (ER)
(3) 17a-hydroxylase
(ER)
(4) 21-hydroxylase (ER)
(5) 11b-hydroxylase
(Mitochondria)
Control of Glucocorticoid (Cortisol) Synthesis
Cortisol: functions to increase blood sugar through gluconeogenesis, to suppress the immune system, and to aid in the metabolism of lipids, proteins, and carbohydrates.
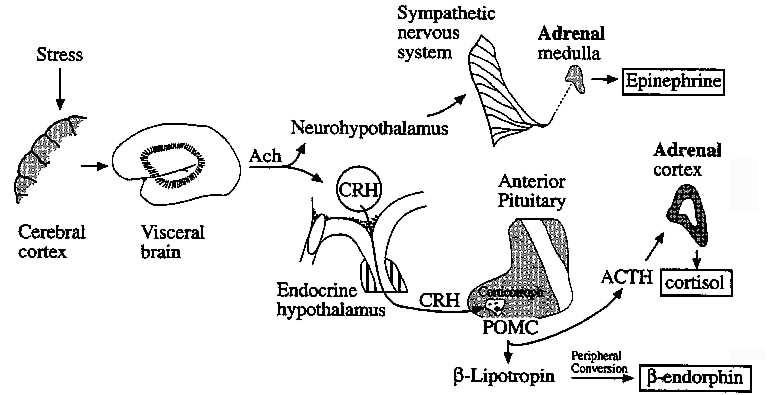
Neuroendocrine Pathways in Emotional Stress Leading to Adrenal
Activation
CRH: Corticotropin Releasing Hormone
POMC: Proopiomelanocortin, complex hormone
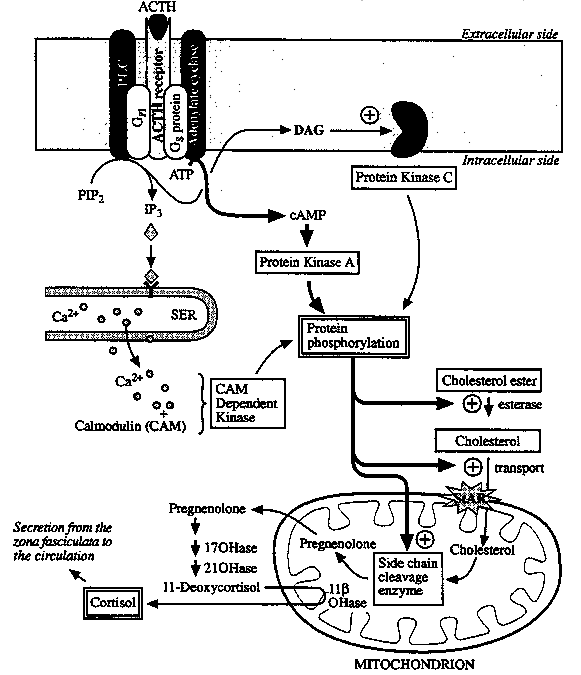
Proposed Mechanism of Action of ACTH in the ZF of the Adrenal
Cortex:
Clinical Correlate:
Cushing's Syndrome
© Dr. Noel Sturm 2019






