Glucose Homeostasis and Starvation
Glucose Homeostasis: the balance of insulin and glucagon to maintain blood glucose.
Insulin: secreted by the pancreas in response to elevated blood glucose following a meal.
Insulin lowers blood glucose by increasing glucose uptake in muscle and adipose tissue and by promoting glycolysis and glycogenesis in liver and muscle.
Insulin:Glucagon Ratio: everything that happens to glucose, amino acids and fat in the well fed state depends upon a high insulin to glucagon ratio.
Glucagon: a fall in blood glucose increases the release of glucagon from the pancreas to promote glucose production.
Glucose Tolerance Test: evaluates how quickly an individual can restore their blood glucose to normal following ingestion of a large amount of glucose, i.e. measures an individuals ability to maintain glucose homeostasis
Diabetic: can not produce or respond to insulin so thus has a very low glucose tolerance
Glucose, Protein and Fat Pathways:

Obese Individuals: even with prolonged medically supervised fasting have plasma glucose levels that remain relatively constant even after three months.
Glucose / Fatty Acid / Ketone Body Cycle:
"explains the reciprocal relationship between the oxidation of glucose versus fatty acids or ketone bodies"
Principal Hormone Effects on the Glucose-Fatty Acid Cycle:
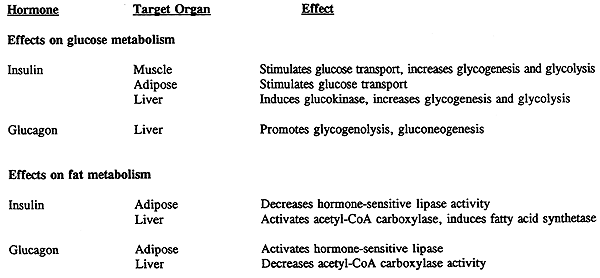
Under conditions of CHO stress (lack of CHO's):
There is depletion of liver glycogen stores
Fatty acids are mobilized from adipose and their rate of oxidation by muscle is increased, which in turn decreases glucose utilization.
Glucagon signals fat mobilization.
Under conditions of plentiful CHO's:
Fatty acid release by adipose is reduced by insulin, thus decreasing fatty acid oxidation.
Glucose use by the muscles increases.
These responses stabilize blood glucose.
The regulatory effect of fatty acid oxidation on glucose utilization is logical:
1) the small reserves of CHO in the body
2) the obligatory requirement by some tissues (i.e. brain, RBC) for glucose
In muscle: fatty acid oxidation decreases glucose utilization through negative effects on glucose transport as well as on the activities of hexokinase, PFK-1 and pyruvate DH
Elevated levels of plasma fatty acids increase muscle oxidation of this fuel.
Ketones: produced from excess fatty acids, provide an alternate fuel and limit glucose oxidation in a similar way as fats, even in the brain.
Glucose / Fatty Acid / Ketone Cycle (pancreas, liver, muscle, adipose, brain):
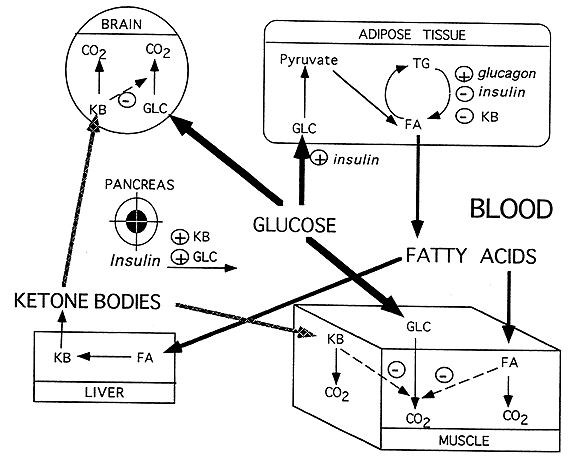
FA= Fatty Acid; GLC= glucose; KB= Ketone Body; TG= Triacyglycerol
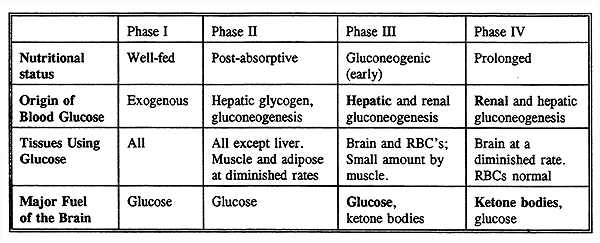
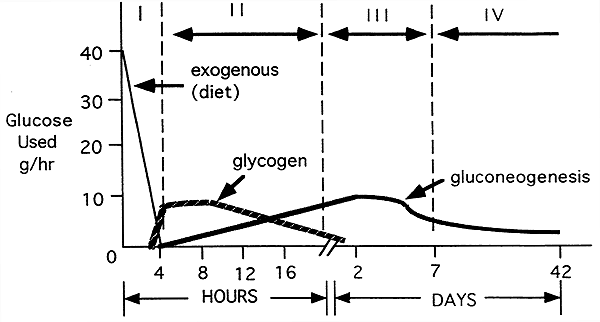
The Four Phases of Glucose Homeostasis:
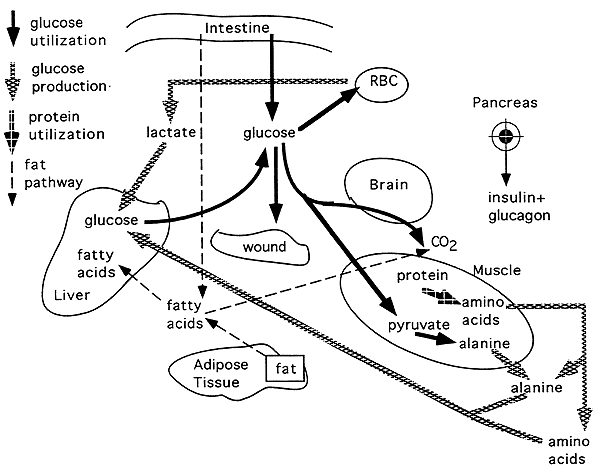
Disposition of Glucose and Fat by Various Tissues in the Well-Fed State (Phase I):
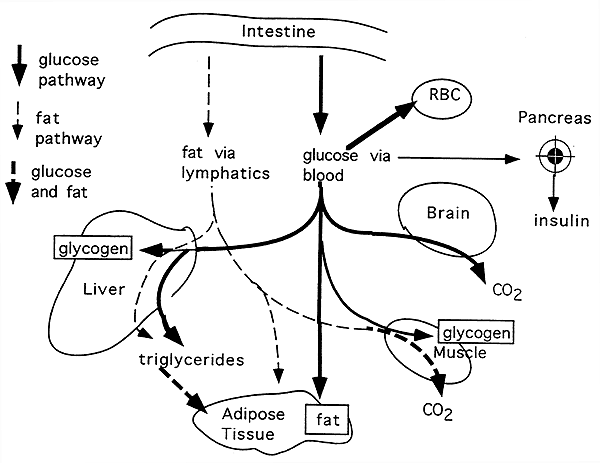
The well-fed state operates while food is being absorbed from the intestine.
CHO and fat are oxidized to CO2 and H2O in peripheral tissues to drive synthetic reactions and sustain cell function.
After a meal, increased plasma glucose promotes the release of insulin and surplus fuel is converted to glycogen and fat.
In the liver, glucose can be converted into glycogen or pyruvate, or pentoses for the generation of NADPH for synthetic processes.
Pyruvate derived from glucose can be used for lipogenesis.
Much of the absorbed glucose circulates to other tissues.
The brain is dependent upon glucose catabolism for its production of ATP.
The liver utilizes glucose and does not engage in gluconeogenesis, thus the Cori cycle is interrupted.
The liver lets most of the amino acids pass through, this is especially important for certain essential amino acids needed by all tissues for protein synthesis.
Excess amino acids not needed for protein synthesis are converted to glucose or fat, with the amino nitrogen going to urea.
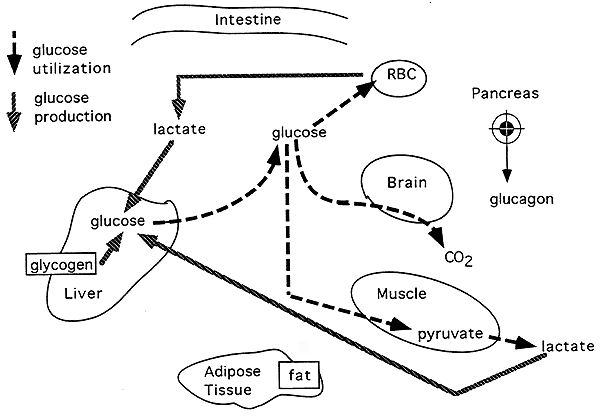
In the postabsorptive phase, liver glycogenolysis provides the most glucose (75%) with gluconeogenesis providing the remainder (alanine 5-10%; lactate 10-15%).
The glucose-alanine cycle is becoming active.
50-60% of glucose is consumed by the brain.
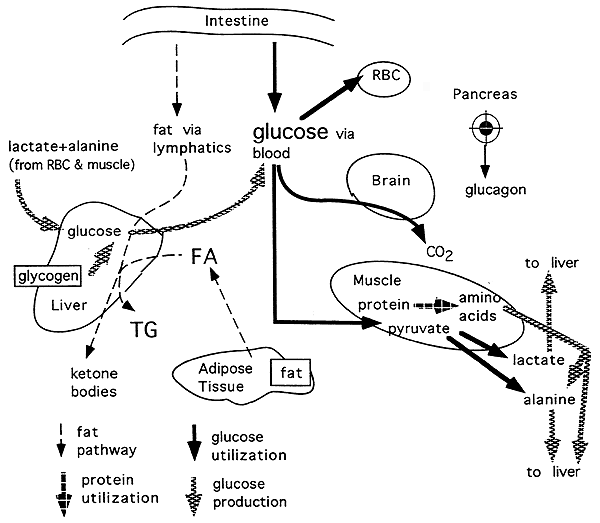
Glucose Production and Utilization in Phase II, the Postabsorptive Phase:
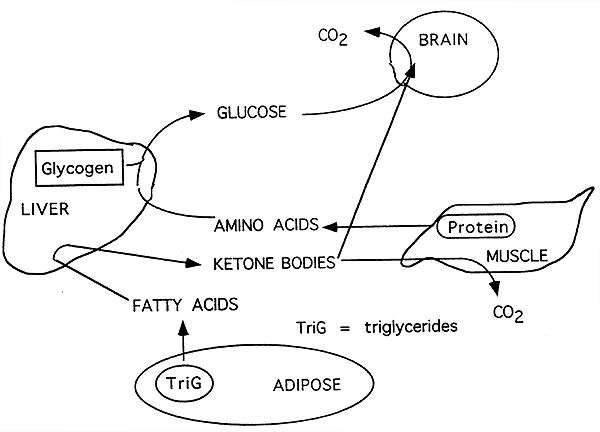
The Gluconeogenic (Early) Starvation Phase (Phase III) :
These phase is characterized by events which occur 24 to 72 hours after the last meal.
The brain still depends solely on glucose but other peripheral tissues begin to switch to fatty acids.
The glucose-fatty acid cycle is starting to switch its emphasis to free fatty acids as fuel.
Dietary fuel is unavailable and no liver glycogen remains to maintain blood glucose.
There is complete dependence upon hepatic gluconeogenesis, primarily from lactate and alanine.
Fatty acids cannot be used for the net synthesis of glucose.
Proteins must therefore by hydrolyzed within muscle to produce amino acids for glucose synthesis in liver.
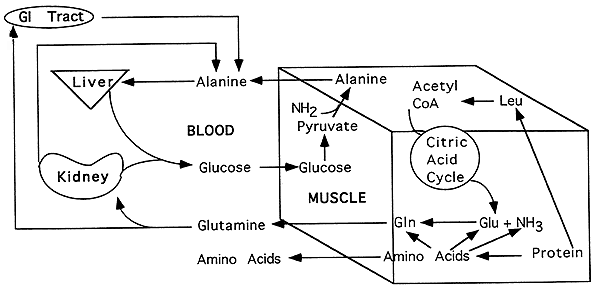
Fate of Amino Acids From Muscle Protein Breakdown in Starvation, Phase IV:
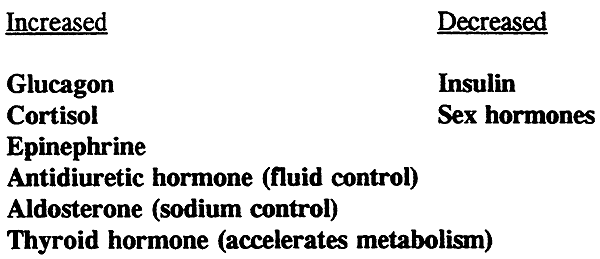
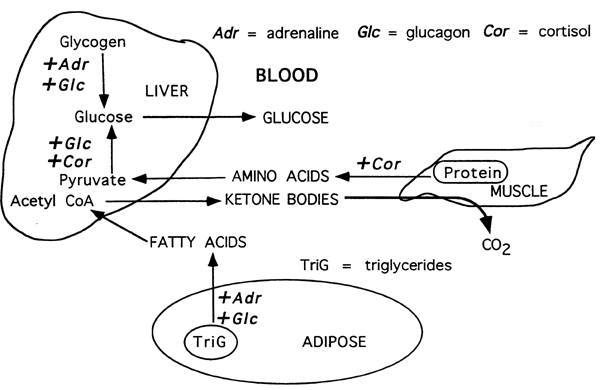
Role of Hormones in Response to Starvation and Stress:
Prolonged Starvation, Phase IV:
Ketones play a central role in prolonged starvation, replacing glucose as the primary fuel for the brain and signaling a reduction in protein catabolism and alanine output from muscle.
Protein conservation is achieved and glucose homeostasis is maintained.
Trauma: