Purine Metabolism
The Bases
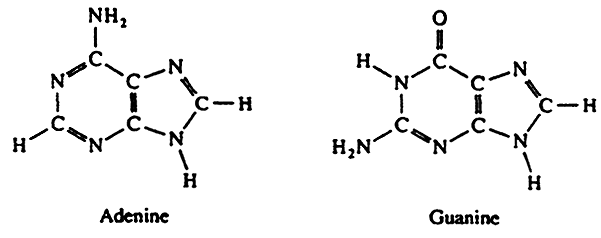
Purines:

Nucleotide Composition
| Base | Ribonucleoside |
Ribonucleotide |
| (Base + Ribose) | (Base + Ribose + Phos.) | |
| Adenine(A) | Adenosine | Adenosine 5'-monophosphate(AMP) |
| Guanine(G) | Guanosine | Guanosine 5'-monophosphate(GMP) |
| Cytosine(C) | Cytidine | Cytidine 5'-monophosphate(CMP) |
| Uracil(U) | Uridine |
Uridine 5'-monophosphate(UMP) |
Ribose:
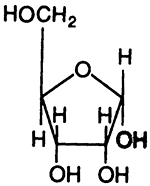
A "Ribonucleoside", A "Deoxyribonucleoside", A "Ribonucleotide"
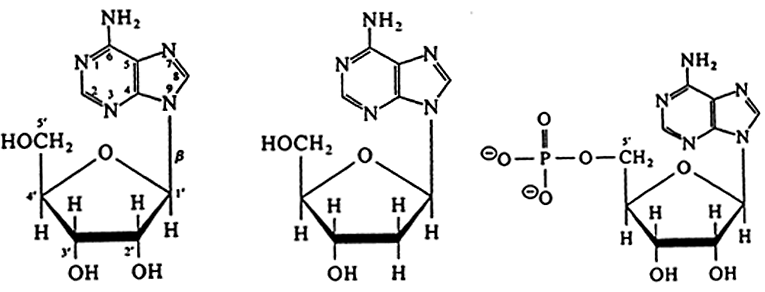
AMP:
ADP:
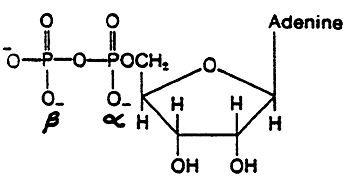
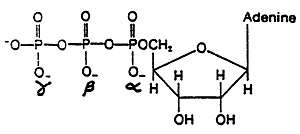
ATP:
Purine Biosynthesis
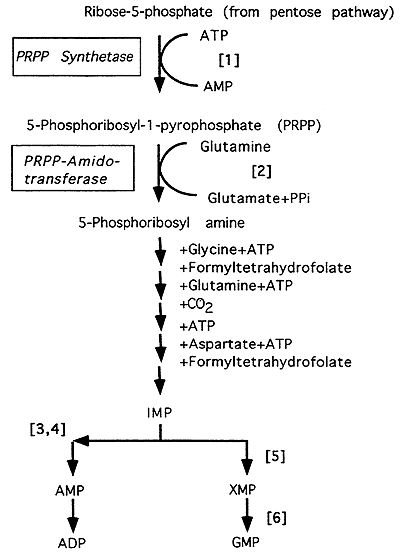
[1] 5-Phosphoribosyl-1-pyrophosphate (PRPP) synthesis is catalyzed by PRPP synthetase. Note: the ribose-5-phosphate for the pathway comes from the Pentose Phosphate Pathway (see "PPP/Gluconeogenesis" Lecture).
[2] The committed, regulated step in the pathway catalyzed by PRPP amidotransferase. Note: glutamine provides the nitrogen to initiate purine synthesis.
Inosinic acid (IMP) is the first purine formed, overall 6 high energy phosphate bonds are consumed to produce IMP.
[3] IMP + Aspartate + GTP ---> Adenylosuccinate; Enzyme: Adenylosuccinate Synthetase
[4] Adenylosuccinate ---> AMP + Fumarate; Enzyme: Adenylosuccinase
[5] IMP + NAD+ ---> XMP + NADH + H+; Enzyme: IMP Dehydrogenase
[6] XMP + Glutamine + ATP ---> GMP + Glutamate + AMP + PPi
Regulation of Purine Biosynthesis
The PRPP amidotransferase enzyme exists as an active monomer and an inactive polymer (see "Introduction to Metabolism" Lecture). IMP, GMP and AMP all inactivate the enzyme causing a shift towards the polymerized inactive form. PRPP causes a shift towards the active monomeric form.
AMP is a competitive inhibitor (see "Enzymes:Catalysis and Kinetics" Lecture) of Adenylosuccinate Synthetase, GMP competitively inhibits IMP Dehydrogenase. Note: GTP is required for AMP synthesis and ATP is required for GMP synthesis, hence there is coordinated regulation of these nucleotides.
Purine Degradation
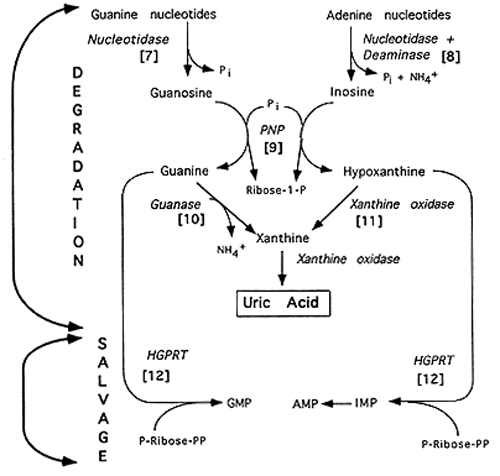
Note: PNP = Purine Nucleotide Phosphorylase; HGPRT = Hypoxanthine-Guanine Phosphoribosyl Transferase
[7] Nucleotidase: Purine Mononucleotide + H2O ---> Purine Nucleoside + Pi
[8] Deaminase: Adenosine + H2O ---> Inosine + NH4+
[9] Purine Nucleoside Phosphorylase: Inosine + Pi ---> Hypoxanthine + R-1-P; Guanosine + Pi ---> Guanine + R-1-P; Note: R-1-P can be converted to R-5-P
[10] Guanase deaminates guanine to xanthine
[11] Xanthine Oxidase oxidizes hypoxanthine to xanthine, then
xanthine to uric acid which is excreted in the urine. Co-factors: Mo, Fe3+,
FAD, O2.
Note: H2O2, a product of this reaction
must be scavenged by Glutathione Reduced (see "PPP/Gluconeogenesis"
Lecture)
[12] Purines can be salvaged after steps [7] and [8] by reconverting
the nucleosides to nucleotides via nucleotide kinases. The free base can also
be saved in a salvage pathway consisting of a single enzyme, HGPRT:
Hypoxanthine + PRPP ---> IMP + PPi; Guanine + PRPP ---> GMP + PPi
.
The importance of this pathway as a source of nucleotides is shown by the consequences
of a deficiency of HGPRT known as Lesch-Nyhan syndrome. This pathway reduces
the need and energy expenditure of biosynthesis by maintaining nucleotide levels.
Clinical Correlates
Gout: is associated with either increased formation of uric acid or its decreased renal excretion. Its incidence is relatively high, occurring in about 0.3% of the population. Gout produces a painful arthritis, particularly in the joints of the extremities. There are two broad types of gout: primary and secondary. Primary gout, of which there are several types, is inherited. Secondary gout is brought on by a variety of disorders such as leukemia.
Individuals with a glucose-6-phosphatase (G-6-P ---> Glucose, in the liver) deficiency develop a glycogen storage disease. This condition results in hypoglycemia, low blood sugar, accumulation of lactic acid and ketones. Distal kidney tubule excretion of uric acid is inhibited and hyperuricemia (XS uric acid) and gout results.
Treatment: the drug that most effectively inhibits the formation of uric acid is allopurinol, a competitive inhibitor of xanthine oxidase. Hypoxanthine and xanthine are excreted during allopurinol therapy. Allopurinol, as with guanine and hypoxanthine, can be converted to its ribonucleotide form by HGPRT. Reducing the formation of uric acid with allopurinol relieves the symptoms of gout and decreases the possibility that uric acid kidney stones will form.
Lesch-Nyhan Syndrome: produces a tremendous overproduction of uric acid. The defect in Lesch-Nyhan disease is the gene for the enzyme HGPRT. The lack of HGPRT activity is virtually complete and the disease is X-linked recessive and thus limited to males. It has a very early age of on-set and is characterized by extremely aggressive behavior that generally leads to self-mutilation. Initially HGPRT was considered a minor player in the "salvage" pathway that permitted reutilization of purine bases. The severity of Lesch-Nyhan syndrome, however, suggests a more important role. Most likely the enzyme has an essential role in non-hepatic tissues where biosynthesis of purines occurs at a very low rate. Non-hepatic tissues contain the enzyme but depend on circulating purines and nucleosides to form nucleotides.
© Dr. Noel Sturm 2020