and
All materials directly linked to this Web page, accompanying photos and videos are in the public
domain and may be copied without restriction.
by Ulrich de la Camp and Oliver Seely
INTRODUCTION
Crude sodium carbonate, Na2CO3, is commonly called soda ash.
It is frequently used as a
commercial neutralizing agent. Besides the carbonate small amounts of sodium hydroxide,
NaOH, and sodium hydrogen carbonate, NaHCO3, may also be present. Titrating
with standard
acid, usually HCl, makes it possible to determine the total alkalinity of the soda ash. It is
common
practice to report the total alkalinity as percent sodium carbonate or sodium oxide,
Na2O. Since
samples are frequently non-homogeneous, the method of aliquot portions is usually employed.
Instead of weighing out three separate samples of soda ash, one accurately weighs out a larger
amount. This is then transferred into a volumetric flask, dissolved in water and then diluted to an
accurately known volume. From this solution are then taken samples or aliquots on which the
titration is carried out.
DISCUSSION
The titration involved in the determination of the carbonate content is an example of a weak base being titrated with a strong acid. The two weak bases involved are:
![]()
and
![]()
The reactions involved are:
![]()
and
![]()
The equivalence point pH for
reaction (1) occurs at a pH of about 8.3, hence a suitable and commonly used indicator is
phenolphthalein. The equivalence point for reaction (2) occurs at a pH of roughly 4.0. Indicators
that have been used are methyl red, methyl orange, methyl purple and bromocresol green.
Bromocresol green will be used in the analysis that you will perform. At the beginning of the
titration, CO32- exists to the practical exclusion of the other carbonate
species. When one equivalent of acid has been added, almost all of the
CO32- has been changed
into HCO3-. Addition of a further equivalent of acid changes
practically all hydrogen carbonate
into carbonic acid, H2CO3. The latter is in equilibrium with water
and
CO2. The steep portions of
the titration curve near the two equivalence points are not so steep and do not extend over so
large a pH range as is required for a titration accuracy of 0.1 relative percent.
Near the HCO3- equivalence point pH of 8.3 the change in pH
caused by adding 1.0 mL of acid is
only about 0.3 units and 10 mL are needed for a pH change of 1 unit. The situation near the
second equivalence point at pH 4.0 is somewhat more favorable. About 4 mL of acid are needed
for a pH change of 1 unit. The accuracy of the titration can be improved considerably by removal
of the CO2 just before the second equivalence point has been reached. An accuracy
of better than
0.1 relative percent may be obtained by the experimental procedure outlined below.
Phenolphthalein indicator is added to the carbonate solution which is then titrated with HCl
until
the pink color has just disappeared, which will occur at an approximate pH of 8. Since the
equivalence point is at pH 8.4 an amount of acid somewhat in excess of one equivalent is used,
and the titration gives only a rough measure of the carbonate content of the original solution.
Next bromocresol green indicator is added, which will turn the solution blue. When the titration
is continued the solution will gradually turn from blue to green and will then approach yellow.
Just before the solution turns completely yellow the solution is boiled to remove the dissolved
CO2. This will change the pH back to somewhere between 8 and 9 and the
indicator
color back
to blue. The solution is then cooled in an ice bath and the titration is continued until the solution
becomes yellow-green. Removal of the CO2 changes the aspects of this last part of
the
titration to one of a
strong base titrated with a strong acid, which explains the accuracy of better than 0.1% that can
be obtained by this method.
The complete equation for this reaction, and the one on which you will base your calculations, both for the standardization of HCl and the determination of the mass % Na2CO3 in soda ash is
Since anhydrous sodium carbonate of high purity can readily be obtained, it is often used as a
primary standard for the standardization of strong acids. You will use this reagent for your
standardization, however, be certain that you are using the anhydrous reagent and not the
decahydrate.
EXPERIMENTAL
Preparation and Standardization of 0.10 M HCl
Dry about 2.0 g of primary standard, anhydrous Na2CO3, in a
weighing bottle for 2 hours at
105-110C, then cool and store in a desiccator.
Rinse your 1-quart glass bottle with distilled water. Add distilled water just up to the beginning of the gentle curve near the top, leaving a little air between the water surface and the neck. Using an appropriate graduated cylinder, measure and add 17 mL 6M HCl. Then add a little water to the graduated cylinder, swirl gently and add the mixture to the bottle. Stopper the bottle and invert several times to achieve a homogeneous mixture. One quart is 946 mL, almost one liter. Seventeen mL of 6M acid when diluted with 946 mL water gives a solution equal nearly to 0.1M concentration:
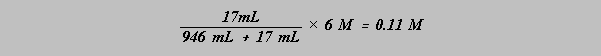
This solution is to
be standardized using anhydrous Na2CO3 so there is no need to attend
to
analytical precision in this procedure. The concentration of commercial HCl isn't assayed with a
precision greater than ±0.2M (it is routinely assayed at 36.5-38.0%, close to 12 M) so even
if the 17mL were measured to ±0.001 mL the molarity still couldn't be
determined with a precision sufficient for this experiment. To achieve the necessary precision
the
solution must be
standardized with measured samples of anhydrous sodium carbonate.
Accurately weigh out three 0.20 to 0.25 g samples of dried, anhydrous Na2CO3 directly into separate, clean and dry 250 mL Erlenmeyer flasks. Add 50 mL of distilled water to dissolve the carbonate, then cover the flasks with parafilm. Rinse your clean 50 mL buret with small portions of your HCl solution and then fill it with the acid solution. Record the initial reading (which does not have to be exactly 0.00 mL) to the nearest hundredth of a mL. Anhydrous sodium carbonate absorbs both water and carbon dioxide from the atmosphere, so your samples will likely show a slow increase in weight. It is not unusual to observe an increase of 0.0001 g every 5 to 10 seconds. You musn't dilly dally in trying to make the mass equal to 0.2000 g or 0.2500 g or some other specific number, or the gradual increase in mass will produce a significant systematic error in your results. Transfer the reagent until the observed mass is between 0.2 and 0.25 g, close the doors of the balance, take a reading when the mass stabilizes to ±0.0001 g and be done with it. One other warning: crystals of anhydrous sodium carbonate don't stick together on your flat spatula. They tend to fall off so during your transfers don't try to make large mounds of the reagent on your spatula tip or you'll lose some. The places where you don't want to lose any is down the side of the flask wall, on the lip of the flask or on the pan outside the flask. You want all of it to go into the mouth of the flask.
The titration which is described below involves a blue-to-green color transition. The subtlety
of
the change is not easily seen by some people. Blue-green color blindness is common in our
population and there are many people who are unaware that they suffer a slight impairment. To
be sure that you can determine a reproducible end point, prepare 100 mL of 0.05 M NaCl in a
250
mL Erlenmeyer flask by diluting 5 mL 1.00 M NaCl to 100 mL with distilled water. Add 3 drops
bromocresol green indicator and 3 drops phenolphthalein indicator, boil briefly, cool and titrate
to
the end point where the green color
just changes to yellow-green. Take care, as the volume required will be quite small, possibly as
little as one drop. This volume is called the "indicator correction", "blank correction" or
"titration
error"; it ought
to be subtracted from your other titration volumes (because the volume is that which is required
to reach the end point for a sample containing no
Na2CO3).
Keep this titration as a guide to repeatability for all future titrations. It gives you a reference
color for your end point. Bringing all future titrations to that final color and then subtracting the
indicator correction from your final volumes ought to improve your results. At the end of every
lab period throw out your indicator correction titration and make up a new one at the beginning
of the next lab period.
Add 3 drops of phenolphthalein indicator to one carbonate sample and titrate it with the acid,
as
described above in the discussion section. Place a piece of white paper under the flask which is
to
be titrated so that you can see easily any subtle color changes. At the first
equivalence point the color will fade quite slowly, therefore do not expect a
sudden change from
pink to colorless. Use your best judgment. Phenolphthalein is colorless in acid solutions and
vivid pink in basic solutions. The first equivalence point of CO32- is
reached when the solution has
changed from pink almost to colorless but still has a ghostly hint of pink left in it. After the first
equivalence point, add 3 drops of bromocresol green indicator; the
solution will turn blue. Titrate with HCl until the solution just begins to change from blue to
green. If it turns yellow you have gone too far. Your solution must be discarded and you must
start over with your second sample. A new "first" sample can be reweighed after the last two
have been completed successfully. Add acid while continually swirling the flask. When the blue
color begins to fade into the green, heat the solution to boiling on a hot plate to expel the
CO2
formed during the titration. Cool to room temperature with the aid of an ice bath. The solution
should again be green, possibly even blue. Complete the titration by adding acid dropwise until
the solution changes to the color of the blank prepared previously. Record the buret reading and
calculate the total amount of acid used from the beginning of the titration to the bromocresol
green end point after boiling. Correct the volume of acid used with the aid of the buret
calibration graph prepared earlier and subtract your end point error, or blank correction. Now
repeat the same procedure with the other two samples one at a time. From the acid
volume and the mass of Na2CO3 used calculate the molarity of the
HCl
solution. The average
deviation from the mean molarity ought not to be greater than 0.2% of the mean.
Titration of a Soda Ash Sample
Dry your unknown sample in an oven for 1 hour at 110C and cool in a desiccator. Using a
weighing bottle, accurately weigh out 2.5 g to an accuracy of ±0.0001 g. Using your wash
bottle
add a small amount of distilled water to the weighing bottle and then transfer the dissolved
material into a clean 250 mL volumetric flask using your wide-stem funnel and water washes.
Add additional distilled water to the volumetric flask and make up to the calibration mark.
While
adding water be sure to mix the contents of the flask well by agitating it. However, do not invert
the flask at this stage. Only after filling to the mark can you invert it. When filling to the
calibration mark use your eye-dropper to add the last few drops.
The next step will include the use of a 25 mL volumetric pipet.
Click here to receive some helpful hints on the use of a volumetric pipet.
When using a rubber bulb to draw liquid into the pipet do not force the bulb over the end of the
pipet. If necessary ask your instructor for help. Once you feel that you are proficient in the use
of pipets, use a 25 mL pipet to transfer three 50 mL aliquots of the unknown solution into three
separate 250 mL Erlenmeyer flasks. Using a 25 mL pipet to measure out these aliquots is
considerably easier than using a 50 mL pipet which, because of its size, is difficult to handle.
Titrate each sample, one at a time, as in the procedure for the standardization of the acid.
Report
From your experimental data calculate, for each aliquot taken, the percentage of
Na2CO3 in the
unknown. Your report must include the following data.
1. Unknown number
2. The mass of each sample anhydrous sodium carbonate
3. Volume of HCl for each sample of anhydrous sodium carbonate
4. Average molarity of the HCl used
5. The mass of your unknown sample.
6. Aliquot volume of unknown solution to be titrated
7. For each aliquot taken give the net volume of acid used to the bromocresol green endpoint (the corrected volume which will be used to calculate the percent sodium carbonate).
8. Percentage of Na2CO3 in the soda ash for each aliquot titrated
9. Average percentage of Na2CO3 in the soda ash
10. Average deviation from the mean of the individual values of percent Na2CO3.
11. Pages in your lab notebook containing the pertinent data
1. Why is the solution boiled just before reaching the second equivalence point?
2. The soda ash in your analysis was assumed to be pure Na2CO3. If some of the Na2CO3 is replaced by an equal number of formula weights of NaHCO3, how would the volume of acid change from (a) the starting point to the phenolphthalein end point? (b) the phenolphthalein end point to the bromocresol green end point? (c) the starting point to the bromocresol green end point?
3. Referring to question 2. above, how would the volumes change if some of the Na2CO3 were replaced with an equal number of formula weights of NaOH?
4. Why does the pink color at the first equivalence point fade only gradually?
5. What is the primary standard which is used for the standardization of the HCl?
6. What is meant by the "aliquot portion method"? Why is it used in this analysis?