Chapter 1, Introduction to Quantitative Chemical Analysis
People are curious about the substances contained in material with which they come in
contact. Precious metals may make them rich, medications may cure diseases and toxins and
poisons may make them ill or worse. Analytical chemistry is the science which helps to satisfy
such curiosity. Qualitative Analysis tries to answer the question "What's in this stuff?" and
Quantitative Analysis tries to answer "How much?"
In 1894 the renowned chemist Wilhelm Ostwald summed up the matter when he wrote
"Analytical chemistry, or the art of recognizing different substances and determining their
constituents, takes a prominent position among the applications of science, since the questions
which it enables us to answer arise wherever chemical processes are employed for scientific or
technical purposes. Its supreme importance has caused it to be assiduously cultivated from a
very
early period in the history of chemistry, and its records comprise a large part of the quantitative
work which is spread over the whole domain of science."
Today in routine medical examinations a single sample of blood can yield quantitative
information on several dozen constituents. Quantitative analyses are used in one form or another
in the processing of all raw materials, from the determination of carbon, nickel and chromium to
determine the hardness of steel to the analysis of sugar content of grapes hour by hour near the
time of harvest so as to give the vintner the best quality grape for fermentation into wine.
This class will offer you some experiences with four methods of quantitative analysis:
gravimetric,
acid-base volumetric, iodometric volumetric and spectroscopic. Some other methods, certainly
equally as important as the ones with which you will come in contact ought at least to be
mentioned:
mass spectrometry for the determination of the masses of molecular fragments and their
quantities,
radioactivity for the determination of the abundance of certain isotopes, heat and rate of reaction,
thermal conductivity, optical activity and refractive index.
Doing an Analysis
The process of doing an analysis involves a number of steps which might not be done in the
sequence shown, but are important points to consider and usually to execute in any analysis:
Deciding on an analytical method
Collecting and preparing the bulk sample(s)
Preparing the lab sample
Defining samples to be analyzed (replicate samples)
Preparing solutions of a sample
Paying attention to properties of the sample matrix so as to be able to eliminate interferences
Calibration, measurement and calculating results
Evaluation of results and estimation of reliability
1. The field of analytical
chemistry is so
very important in commerce that money is often involved and cost and profits are routinely
considered. The cost of an analysis usually goes up with the level of accuracy demanded so it is
not
surprising that the method may be a compromise between accuracy and economics. The method
to
be used also is a function of the number of components in the sample which may interfere with a
certain analysis.
2. The decision of how to collect and to prepare your bulk samples depends sometimes on
what it is that one wants to demonstrate. Pfiesteria piscicida, a single-celled
microorganism which lives in the
Chesapeake Bay and other bodies of water near the Virginia and North Carolina coasts, is known
to thrive where there is an overabundance of nutrients such as nitrogen and phosphorus in the
water. These include nutrient-rich human sewage, fertilizers, certain industrial by-products and
animal wastes from swine and poultry. Such an environment allows algae to proliferate and the
algae provide a rich food source for Pfiesteria. In 1991 a billion fish died in the
Neuse Estuary of North Carolina as the result of succumbing to an agent or an effect connected
with an organism in the food chain of which Pfiesteria is one. Some say
Pfiesteria produces a powerful toxin which kill the fish. Others suggest that the fish
become part of the food chain and are eaten by the microorganisms. The matter is further
complicated by evidence which suggests that some but not all varieties of a species of algae
produce toxins and studies of the life cycle of Pfiesteria have produced ambiguous
results. Proponents of one hypothesis will more likely than not defend their position vigorously
in
the face of evidence which other investigators find to be less than convincing. Periods of "red
tide" along much of the coastline of the U.S. in which shellfish cannot be eaten because of their
toxin content further complicate finding the source of natural products which are toxic to
humans;
moreover, one researcher claims that agricultural and industrial wastes are not the cause of algal
blooms but the fault lies in red sand blown across the Atlantic Ocean from the Sahara Desert
which triggers the algal blooms in the mid-Atlantic. As for the Pfiesteria
controversy,
more than a decade has passed and the matter seems to be nowhere near resolution. One would
want to choose carefully how samples are to be taken based on whatever hypothesis it was to be
tested. If the nutrient-
algae-Pfiesteria link was a hypothesis to be supported, then water samples would
have to be tied to a program of culture growth of the microorganism. If the link was felt to be
well-established and the analysis was a part of an ongoing monitoring program then one might
concentrate on regions where high nutrients might be expected to be found throughout the year.
If
the purpose of the analysis was a part of a program to protect the fish population then the
samples
might be taken during only certain months.
If a large quantity of precious metal ore is to be analyzed for the metal before a price can be
agreed
upon, one would definitely want to take samples from locations within the shipment at points of
some
wide separation so as to be able better to determine the variability of the metal within the
shipment.
Ores, which are commonly heterogeneous, require prudent choices to obtain representative
samples.
3. Preparing the lab sample. Homogeneity of each sample taken from the larger bulk sample
must
be achieved at this point. Grinding and drying may be involved at this step. The determination
of
the
amount of H2O may be required as a part of the overall analysis. The grinding
process not only
achieves homogeneity but gives the technician a sample with finely divided particles for later
work
in producing a solution.
4. Defining the samples to be analyzed (replicate samples). Replication improves reliability
because the variance between
individual samples of the determined quantity of analyte establishes a level of reliability of the
method
used. Often individual quantities are weighed and those quantities become the laboratory
samples; the process of analysis starts after this weighing. Sometimes the grinding operation is
found
not
to produce a bulk sample sufficiently homogeneous to use this method. In this case then a larger
sample may be weighed and that sample then dissolved in a measured volume of appropriate
liquid.
This process assures homogeneity of the analyte and measured volumes of the solution are then
taken
as the lab samples.
5. Preparing solutions of a sample. Most analyses are done on solutions because the
analytical
reactions go at greatest speed when the analyte is divided down to the atomic, ionic or molecular
level. All of the sample must be dissolved so as to assure that none of the analyte has occluded
onto
the surface or within the crystal lattice of any insoluble residue. Unfortunately, this step may be
time-consuming but must be followed so as to exceed any reasonable doubt about the state of the
analyte.
6. Paying attention to the properties of the sample matrix so as to be able to eliminate
interferences. The families of elements found in the Periodic Table attest to
there being
few elements with unique properties. Thus, in practically all analyses there can be many
interferences.
Lead precipitates as the sulfide, but so does copper, mercury, nickel and silver. The oxidized
forms
of manganese (permanganate) and chromium (dichromate) have useful absorption spectra in the
visible region. Both of these may be present in small amounts in steel. But iron which is present
in
the largest amount in steel oxidizes to Fe3+ and has an absorption spectrum in the
same region. Citrus
fruits contain a large number of natural acids which would preclude the analysis for any one of
them
using simple acid-base titrimetry. It is often necessary that subtle differences in the chemistry of
elements within families be exploited so as to eliminate all interferences from any analysis.
7. Calibration, measurement and calculating results. The measurement which leads to the
final result
is usually directly proportional to that result. In the gravimetric determination of sulfate, all of
the
sulfate in the sample precipitates as barium sulfate, BaSO4. The sulfate, the
SO42-, is the amount one
wishes to calculate, but the weight of BaSO4 is the measured quantity. In many
cases for quantitative
analyses, the calculation takes on the simple form
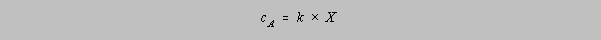
cA is the
desired quantity, the mass of SO42- in 100 g of sample, but X, the
weight of
BaSO4 is that
which is measured.
k is the proportionality constant which relates one to the other, in this case the ratio of the atomic
weight sum for SO42- divided by the atomic weight sum for
BaSO4.
Often one cannot so easily
calculate k; in the case of the colorimetric determination of manganese in steel, the absorbance of
a
known concentration of manganese must be measured. One then has
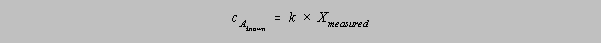
and k can be
calculated from the absorbance of the known concentration by the quotient of cA
and
X. The assumption is that the proportionality holds for all solutions of Mn prepared in the same
way,
both known and unknown. A value of X for an unknown sample will yield a calculated value for
the
concentration of Mn in that sample.
8. Evaluating results and estimating reliability. The announcement of an experimentally
determined value alone
without some indication of its reliability has no scientific worth. In experimental science we
have
a
means for implying some level of reliability: significant figures. As imperfect as is the use of
significant figures the method does at least set some rather broad boundaries of reliability.
Reporting
a value of 2.50% Cr in a sample of stainless steel might suggest a maximum experimental
deviation anywhere between ±0.01% and ±0.09%. If the experimental deviation
were
found to be between ±0.06% and ±0.09%, then one might consider reporting the
value as 2.5 ±0.1% Cr. If nothing were known about the
reliability of a quantity determined
experimentally there would be no justification for reporting any value of that quantity to any
number
of significant figures. Any report of the measurement would be worthless. It is fortunate that
rarely
is nothing known about the reliability of any experimental
measurement. More often than not the
problem is one of data being taken poorly with bad estimates of their reliability, leading to results
the
validity of which is open to withering criticism.