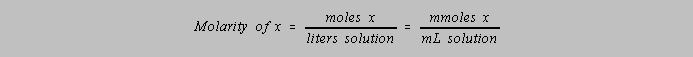
Chapter 2, Basic Concepts
2-1 Système Internationale or SI
A system of units and prefixes established to simplify and to unify communication between
scientists.
| Seven basic quantities | Unit | Abbreviation |
| Mass | kilogram | kg |
| Length | meter | m |
| Time | second | s |
| Temperature | kelvin | K |
| Amount of substance | mole | mol |
| Electric current | ampère | A |
| Luminous intensity | candela | cd |
Mass. The kilogram (kg). The kilogram of a particular cylinder of
platinum-iridium alloy, called the
International Prototype Kilogram, which is preserved in a vault at Sèvres, France, by the
International Bureau of
Weights and Measures.
Length. The meter (m). The meter is the distance that light travels
in a vacuum in 1/299 792 458 of a second.
Time. The second (s). The second is the unit of time of the
International System of Units. The definition
adopted at the October 13, 1967 meeting of the 13th General Conference on Weights and
Measures is: "The
second is the duration of 9,192,631,770 periods of the radiation corresponding to the transition
between the two
hyperfine levels of the fundamental state of the atom of cesium 133." The frequency
(9,192,631,770 Hz) which
the definition assigns to the cesium radiation was carefully chosen to make it impossible, by any
existing
experimental evidence, to distinguish the new second from the "ephemeris second" based on the
earth's motion.
Therefore no changes need to be made in data stated in terms of the old standard in order to
convert them to the
new one. The atomic definition has two important advantages over the previous definition: (1) it
can be realized
(i.e., generated by a suitable clock) with sufficient precision, ± 1 part per hundred billion
(1011) or better, to meet
the most exacting demands of modern metrology; and (2) it is available to anyone who has access
to or who can
build an atomic clock controlled by the specified cesium radiation. (A description of such clocks
is given in
"Atomic Frequency Standards," NBS Tech. News Bull. 45, 8-11 (Jan., 1961). For
more recent developments and
technical details, see R. E. Beehler, R. C. Mockler, and J. M. Richardson, "Cesium Beam Atomic
Time and
Frequency Standards," Metrologia 1, 114-131 (July, 1965)). In addition one can
compare other high-precision
clocks directly with such a standard in a relatively short time -- an hour or so compared against
years with the
astronomical standard. Laboratory-type atomic clocks are complex and expensive, so that most
clocks and
frequency generators will continue to be calibrated against a standard such as the National
Institutes of Standards and Technology (NIST) Frequency
Standard,
controlled by a cesium atomic beam, at the Radio Standards Laboratory in Boulder, Colorado. In
most cases the
comparison will be by way of the standard-frequency and time-interval signals broadcast by NBS
radio stations
WWV, WWVH, WWVB, and WWVL. There has been a recent proliferation of desk and wall
clocks which tune
into these stations a couple of times a day and reset themselves. Often they operate on an AA
battery. When
they first arrived on the mass market they cost around $400, but as in all things "hi-tech" the
prices have fallen.
Desk models can now be purchased for under $30. Come in to your instructor's office and see
the
wall-mount
version.
Temperature. The Kelvin (K). The Kelvin, the unit of
thermodynamic temperature, is the fraction 1/273.16 of
the thermodynamic temperature of the triple point of water. The decision was made at the 13th
General
Conference on Weights and Measures on October 13, 1967 that the name of the unit of
thermodynamic
temperature would be changed from degree Kelvin (symbol:
oK) to kelvin (symbol: K). The name
(kelvin) and
symbol (K) are to be used for expressing temperature intervals. The former
convention which expressed a
temperature interval in degrees Kelvin or, abbreviated, deg. K is
dropped.
However, the old designations are
acceptable temporarily as alternatives to the new ones. One may also express temperature
intervals in degrees
Celsius.
Amount of substance. The mole (mol). The mole is the number of
atoms of C12 in 12.0000 g of C12.
Electrical current. The ampère (A). The ampère
(unit of electric current) is the constant current which, if
maintained in two straight parallel conductors of infinite length, of negligible circular sections,
and
placed 1
meter apart in a vacuum, will produce between these conductors a force equal to 2 x
10-7 newton per meter of
length.
Luminous intensity. The candela (cd). The candela is the luminous
intensity, in the direction of the normal, of a
black body surface 1/600,000 square meter in area, at the temperature of solidification of
platinum
under a
pressure of 101,325 newtons per square meter.
Prefixes vary from yotta (factor of 1024) to yocto (factor of 10-24)
Exponent Prefixes
| Factor | Prefix | Symbol |
| 1024 | yotta | Y |
| 1021 | zetta | Z |
| 1018 | exa | E |
| 1015 | peta | P |
| 1012 | tera | T |
| 109 | giga | G |
| 106 | mega | M |
| 103 | kilo | k |
| 102 | hecto | h |
| 101 | deka | da |
| 10-1 | deci | d |
| 10-2 | centi | c |
| 10-3 | milli | m |
| 10-6 | micro | µ |
| 10-9 | nano | n |
| 10-12 | pico | p |
| 10-15 | femto | f |
| 10-18 | atto | a |
| 10-21 | zepto | z |
| 10-24 | yocto | y |
These are the seven fundamental base units and
accompanying
prefixes. All of the other units may be derived
from these -- they are called derived units.
Thus, 1mL = 1 cm3 and 1 Newton = 1 (kg m)/s2
2-2 The mole
A mole of molecules is Avogadro's number of molecules. Avogadro's number is the number of
12C atoms in 12.0000 g of 12C and has been experimentally
determined to equal 6.022 x 10 23.
The molar mass is the mass of one mol of a substance.
Exercise 2-1a. Determine the molar mass of acetic acid, CH3COOH, .
Add up the atomic weights multiplied by the number of atoms of each element in
the compound to get 60.05 g/mol.
2-3 Calculations in grams and moles
Exercise 2-2a. How many moles of citric acid,
C6H8O7, MW=192.14, are contained in 6.00 g of the
pure acid?
(To be performed in class)
Exercise 2-2b. How many millimoles of citric acid are contained in 6.00 g of the pure acid?
(To be performed in class)
Exercise 2-3a. How many grams Na+ are contained in 32.7 g trisodium
phosphate, Na3PO4 10 H2O, F.W.
344.09?
(To be performed in class)
Exercise 2-3b. How many grams of the phosphate ion, PO43-, are
contained in the amount given above?
(To be performed in class)
2-3. Solutions and their concentrations
The following concentrations and concentration relationships are of importance to and will
often be found in
studies involving the quantitative analyses of chemical substances:
a. Molar concentration.
b. Analytical molarity.
c. Equilibrium molarity of a particular species.
d. Percent concentration.
e. Parts per million/billion (ppm, ppb)
f. Volume ratios for dilution procedures.
g. p-functions.
Molar concentration
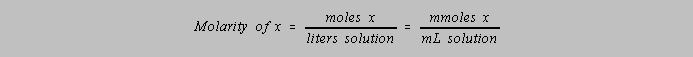
Exercise 2-4. Given a 1.25 liter solution which contains 3.74 g urea,
H2NCONH2, MW=60.06, determine the
molar concentration of urea in this solution.
(To be performed in class)
Regarding solution concentration, one speaks of analytical
molarity, or that which would be present if the
substance does not enter into reactions which change the concentration of the species
under discussion, and
equilibrium molarity of a species which reflects the disappearance
of part of the original species due to
dissociation, complex formation or other equilibria processes.
Exercise 2-5a. Determine the analytical molarity of 11.27 g anhydrous sodium sulfate,
Na2SO4, in 500.00 mL
solution.
(To be performed in class)
Exercise 2-5b. Determine the equilibrium molarity of Na+ and
SO42-, written [Na+] and
[SO42-], in the solution
described above.
(To be performed in class)
Exercise 2-5c. A solution of acetic acid, CH3COOH, MW=60.05, having an
analytical molarity of 0.100 M has
what equilibrium concentration of hydronium ion, H3O+, if the
dissociation goes according to the equation and
the molecular form of the acid is 5% ionized?
CH3COOH + H2O <===> H3O+
+ CH3COO-
(To be performed in class)
Exercise 2-5d. Determine the species molarity of CH3COO- for
the solution described above.
(To be performed in class)
The student of Quantitative Analysis is often asked to prepare a solution of some given
analytical molarity.
Exercise 2-6a. Explain how one would prepare 50 mL of a 0.250 M solution of sodium sulfate decahydrate,
Na2SO4 10 H2O, MW=322.19.
(To be performed in class. First the quantity is determined, then one is asked what exactly is
the procedure
which one follows.)
One might be asked the question above in a form which requires the weight of reagent
necessary to produce a
given species molarity:
Exercise 2-6b. Explain how one would prepare 50 mL of a 0.125 M solution of sodium ion,
starting with the
reagent sodium sulfate decahydrate, Na2SO4 10 H2O,
MW=322.19, assuming 100% dissociation of sodium ion.
Percent concentration. Percent concentration may be thought of as parts
per hundred.
There are three forms of percent concentration which may be encountered:
(1) wt. % (w/w) = (mass solute) ÷ (mass solution) × 100%
(2) volume % (v/v) = (volume solute) ÷ (volume solution) × 100%
(3) wt/volume % (w/v) = (mass solute) ÷ (volume solution, mL) × 100%
Weight percent is often used to express the concentration of commercial reagent grade acids.
Concentrated
aqueous ammonia is sold as 28% (w/w) NH3. (See the Web link
http://www.csudh.edu/oliver/chemdata/acid-str.htm
for a complete listing of properties of commercial acids and bases.)
Volume % often is used where one liquid is diluted by another:
10% aqueous butanol, for example, would be a solution in which 10 mL pure butanol is
diluted to give 100 mL
solution.
Wt/vol solutions are often dilute aqueous solutions in which the calculation involved takes advantage of the fact that the density of water is close to 1 g/mL. Dilute w/v solutions have concentrations very close to the values which would be reported for their w/w concentrations. At higher concentrations there is a greater divergence. For example, 50% w/w NaOH is 76.3% (w/v) NaOH because the density of the solution rises significantly above 1.00 g/mL
The use of (w/w)% for (weight/weight)% is an historical artifact which is still seen from time to time. A better designation would be (m/m)% for (mass/mass)% but because the abbreviation "m" is that which is used for the metric unit of length, (w/w) has stuck around far longer than is justifiable. To get around the use of (w/w), companies which produce reagent grade acids simply say "Assay". That is, on a bottle of Spectrum® reagent grade sulfuric acid one finds the designation "Assay (H2SO4 ). . . . . . . 95.0 - 98.0 %". That designation very definitely describes a percent concentration found by the operation
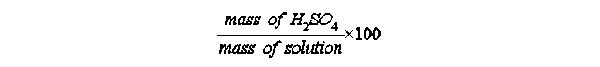
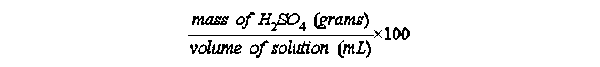
Exercise 2-7. Knowing the above values for the (w/w) and (w/v) solution of NaOH,
determine the density of
that solution.
(To be performed in class)
Consider the following table of solution densities at 20oC:
| Solution | Density |
| 10% (w/w) KCl | 1.08 g/cc |
| 10% (w/w) NaCl | 1.07 g/cc |
| 10% (w/w) KOH | 1.08 g/cc |
| 10% (w/w) KCl | 1.06 g/cc |
| 10% (w/w) BaCl2 2H2O | 1.082 g/cc |
Exercise 2-8. Determine the (w/v) value for the 10% (w/w) solution of KOH above.
(To be performed in class)
Parts per million (ppm). Parts per million is a concentration convention
convenient to use for very dilute
solutions.
Exercise 2-9. Determine the ppm of ferrous ion, Fe2+, in a solution known to be 1.2 × 10-6 M Fe3(PO4)2 8H2O
FW=501.61.
(To be performed in class)
Volume Ratios for Solutions to be Diluted
When diluting solutions, a directive may specify a 1:5 volume/volume dilution. Although
this
usually means 1
volume of concentrated solution to 5 volumes of the final dilute solution, that might not be the
case depending
upon the procedure in any particular laboratory. The student is advised
always to verify whether the meaning
might be 1 volume of concentrated solution to 5 volumes of water. Making a mistake in the
preparation of the
final solution can lead to serious consequences particularly where the preparation of nutrient
solutions in
experimentation on humans and animals is involved.
Exercise 2-10. Determine the volume/volume ratio needed to dilute a 0.5 M saline solution
to
0.2 M.
(To be performed in class)
p-functions The p-function is the negative logarithm to the base 10 of the
molarity of a given species.
Thus, pX = -log[X]
Exercise 2-11a. Given a solution known to be 3.7 × 10-4 M NaCl and 4.5
× 10-3 M HCl , determine pH, pNa and
pCl for these solutions.
(To be performed in class)
Or you may be asked to do the reverse.
Exercise 2-11b. Given a solution with a pOH of 4.37, determine the species molarity of the
OH- ion.
(To be performed in class.)
Notes on logarithmic calcuations:
Today with modern hand calculators, converting X to log10X is a matter of a
single keystroke. Converting a p
function to a concentration is equally simple because of the following relationship.
Exercise 2-12. Consider pAg in a solution of silver nitrate, AgNO3, to equal
6.74. What is the molarity of Ag+ ?
6.74 = pAg = -log10[Ag+ ]
Since the statement y=log10X could be identically rewritten as
X=10y, the expression above could also be
rewritten as
[Ag+ ] = 10-6.74
To convert to molarity, enter -6.74, and press the 10X key, to get
[Ag+ ] = 10-6.74 = 1.82 × 10-7
Density and specific gravity of solutions.
The numeric value for a density given as g/mL or kg/L is the same. Water with a density at
20oC of 0.998 g/mL
has a density of 0.998 kg/L. The density of gases is usually given as g/L because at normal
pressures the density
is about a thousand times less than that of liquids. The density of dry air at 760 torr at 1 atm
pressure is 1.185
g/L.
The specific gravity is a ratio between the mass of a given
volume and the mass of an equal volume of water at
4oC. Since the density of water at 4oC is practically 1.00 g/cc, the
density and specific gravity of aqueous
solutions are almost identical. The difference needs always to be considered for analytical work
of high
precision, however.
Exercise 2-13. Commercial reagent grade formic acid is 90% w/w and has a specific gravity
of 1.20. Determine
the molarity of formic acid in a bottle of commercial reagent grade formic acid.
(To be performed in class)
Given any two of the following three: (w/w)%, molarity and specific gravity, one can
calculate the third. Given
just the molarity, one is able to calculate methods of diluting solutions volumetrically.
Exercise 2-14. Hydriodic acid, HI, is sold in reagent grade bottles as 57% (w/w) HI and has
a specific gravity of
1.70. Explain how you would prepare 100 mL of 1.25 M hydriodic acid, using only a 100 mL
volumetric flask
and a 20 mL graduated pipette.
(To be performed in class)
Stoichiometric calculations.
Stoichiometric calculations follow part or all of the path
1. Mass x ---> 2. Moles x ---> 3. Moles y ---> 4. Mass y
Going from step 2. to step 3. requires a stoichiometric ratio
which is usually determined by balancing a chemical
equation.
Exercise 2-15a. Calcium oxalate, CaC2O4 , can be precipitated by
adding to a solution of calcium hydroxide,
Ca(OH)2, sufficient sodium oxalate,
Na2C2O4 , to react with all calcium hydroxide present.
What mass sodium
oxalate would be required to precipitate all of the calcium in a solution containing 3.74 g
Ca(OH)2?
(To be performed in class)
Exercise 2-15b. What weight of Calcium oxalate, CaC2O4, would
be produced?
(To be performed in class)
Exercise 2-16a. Limiting reagent.
25.0 mL 0.100 M Ca(OH)2 is added to 20.0 mL 15.8 % (w/v)
Na2C2O4. Calculate the weight
CaC2O4 formed.
(To be performed in class)
Exercise 2-16b. What is the equilibrium concentration in molarity of all ionic species
remaining after the
reaction?
(To be performed in class)