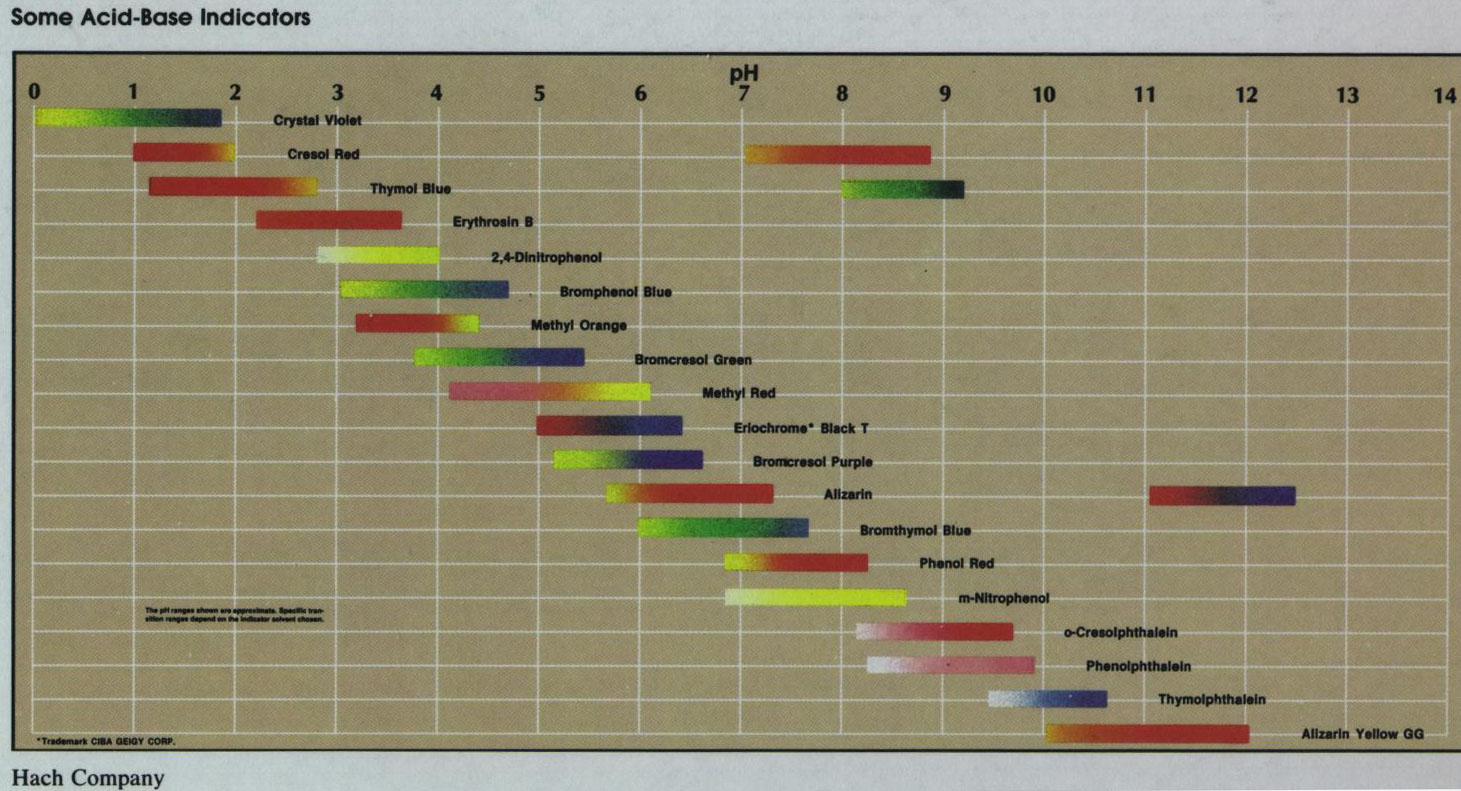
An acid-base indicator is itself an acid-base pair, but one in which the acid form absorbs light
in one part of the visible spectrum and the base form in another. The central point of transition is
the pH at which the acid and base forms occur at equal concentrations. That pH determines the
pK of the
acid form, or the pKa. The full color change occurs over an approximate pH range
of 2, representing a change of [base form]/[acid form] from 1:10 to 10:1. The
figure below shows the transition pH of nineteen common acid-base indicators.