Balances and Their Use in the Chemistry
Laboratory
by Oliver Seely
Updated March 18, 2006
The images shown on this page are supplementary to the information given on the use of
laboratory balances in your text book. This Web page and all direct links on it are in the public
domain and may be copied without restriction.

One of the electronic analytical balances we shall be using is shown at the right.
Your objective is to develop techniques which allow you to transfer samples of material from one
container to another and to have confidence that you know the amounts transferred to a precision
of one tenth of a milligram or one ten-thousanth of a gram, ±0.0001 g.
First, before weighing anything on this analytical balance, it needs to be "tared," or recalibrated
to
read 0.0000 g. When first turned on, or when left by the previous user, the balance may indicate
something other than 0.0000 g. The Tare button needs to be pressed and released to effect this
recalibration. The four images below illustrate this process.




An analytical balance is so sensitive that it can detect the mass of a single grain of a chemical
substance. Thus, if a method of direct weighing is used, the substance ought to be added to the
tared container which will hold it, NEVER directly to the pan or even to weighing paper placed
on the pan. The container used
should be completely dry and at room temperature, never at an elevated or reduced temperature.
Even slight temperature differences can produce APPARENT changes in mass of the container.
Finally, the container ought to be completely dry, inside and out. All that having been said,
here are some images showing various correct ways of carrying out weighings using an analytical
balance.

Regardless of which method illustrated below is used accurately to weigh a sample, the sample,
placed in a weighing bottle set in the upturned cap in a beaker with a watch glass placed on top,
must be first dried in the oven. You may identify your sample by marking the beaker but DO
NOT mark the weighing bottle.

The ovens are kept at 110oC, but our ovens may show slightly lower
temperatures owing to the doors being opened repeatedly during normal laboratory activities.
 The tried-and-true method of transferring a precisely weighed sample uses "weighing by
difference," shown here. The empty balance is tared, then the weighing bottle with cap is placed
on the pan and weighed to ±0.0001 g.
The tried-and-true method of transferring a precisely weighed sample uses "weighing by
difference," shown here. The empty balance is tared, then the weighing bottle with cap is placed
on the pan and weighed to ±0.0001 g.
 The weighing bottle is removed in a manner which avoids the
transference of oil or other matter from one's fingers.
The weighing bottle is removed in a manner which avoids the
transference of oil or other matter from one's fingers.
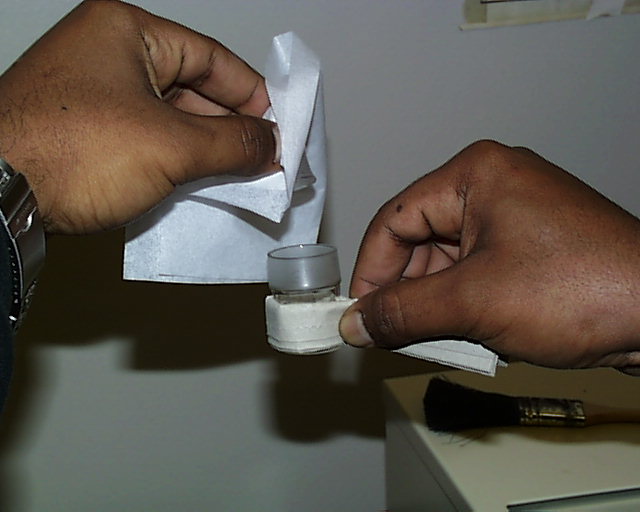 The cap is likewise removed from the weighing bottle.
The cap is likewise removed from the weighing bottle.

 The weighing bottle is tipped above the container to receive the sample and a small amount is
allowed to fall out of the weighing bottle. The weighing bottle is tipped back up and tapped
gently to make sure all of the substance falls back in the bottle and doesn't remain on the bottle
rim. The cap is replaced and the bottle weighed once again. The difference between the first and
second weighings represents the amount transferred. If your
sample has a tendency to absorb water and thus to gain weight when exposed to the moisture of
the air, this method MUST be used to minimize exposure to the atmosphere. Still, the method is
not foolproof and has its own perils: (1) several transfers may be necessary until an amount close
to that needed is
added to the receiving container, (2) too much may be transferred the first time, forcing one to
discard the entire sample ("Drat and blast, I have to start over," is a common epithet heard in
weighing rooms), (3) sample which remains on the weighing bottle rim may be lost and produce
a
weighing error. This peril is repeated each time a transfer is attempted.
The weighing bottle is tipped above the container to receive the sample and a small amount is
allowed to fall out of the weighing bottle. The weighing bottle is tipped back up and tapped
gently to make sure all of the substance falls back in the bottle and doesn't remain on the bottle
rim. The cap is replaced and the bottle weighed once again. The difference between the first and
second weighings represents the amount transferred. If your
sample has a tendency to absorb water and thus to gain weight when exposed to the moisture of
the air, this method MUST be used to minimize exposure to the atmosphere. Still, the method is
not foolproof and has its own perils: (1) several transfers may be necessary until an amount close
to that needed is
added to the receiving container, (2) too much may be transferred the first time, forcing one to
discard the entire sample ("Drat and blast, I have to start over," is a common epithet heard in
weighing rooms), (3) sample which remains on the weighing bottle rim may be lost and produce
a
weighing error. This peril is repeated each time a transfer is attempted.

 The direct transfer of samples to receiving containers is possible using modern analytical
balances
which allow one to tare a receiving container before the transfer is begun. Note the Erlenmeyer
flask on the pan. The Erlenmeyer flask needs to be
completely dry so that there will be no systematic error due to the evaporation of water.
The direct transfer of samples to receiving containers is possible using modern analytical
balances
which allow one to tare a receiving container before the transfer is begun. Note the Erlenmeyer
flask on the pan. The Erlenmeyer flask needs to be
completely dry so that there will be no systematic error due to the evaporation of water.



A spatula can be used to remove a sample from the weighing
bottle and the sample placed
directly into the flask.

This method has some perils associated with it: (1) one risks losing the sample on the
outside of the Erlenmeyer flask due to the small diameter of the flask neck and (2) the point of
entry
is rather high; one must have good coordination to orient the spatula above the mouth of the
flask. Advantage: The sample is placed directly into the flask and the mass read is that of the
sample added. Eternal vigilance being the price of good results, note in the image at the right the
particles of sodium carbonate which didn't quite make it to the bottom of the flask. The titration
yielding the molarity of the hydrochloric acid standard for the percent sodium carbonate in soda
ash experiment showed a precision within one part per thousand with the other titrated samples
but only because both the stopper, which was removed gently before the titration, and the inside
of the flask neck were rinsed carefully to dissolve the particles of carbonate and to allow them to
drain into the body of the flask.
 The mass limit of our analytical balances beyond which one receives an error message is
200.0000
grams. Even this 400 mL beaker can be placed on the pan. Note that its mass is just above 149
g.
The mass limit of our analytical balances beyond which one receives an error message is
200.0000
grams. Even this 400 mL beaker can be placed on the pan. Note that its mass is just above 149
g.
 Once tared, the sample may be placed directly into the beaker, as shown below.
Once tared, the sample may be placed directly into the beaker, as shown below.


The perils using this method are similar to those encountered during a direct transfer to an
Erlenmeyer flask. The beaker must be clean, dry and at the same temperature as the room and
the balance. The mouth of the beaker is rather high, so one must have good coordination to
orient the spatula over the mouth without knocking it against the beaker or the windows of the
balance, thus losing the sample.
 You can still weigh your sample directly but do it in a manner which doesn't require as much care
and coordination as that illustrated above by removing the beaker from the pan, adding your
sample and replacing the beaker on the pan. The advantage of this method is that you have more
room for sample addition and the placement of your hands, arms and the transfer spatula is less
awkward, but the disadvantage is that you must check the weight of your beaker and sample after
each addition; it is not good practice to remove excess sample from the beaker if you add too
much. You must be sure that the removed beaker is placed on a clean and dry part of the bench
so to avoid picking up any particles which will change your weight and decrease your accuracy.
The removal and replacement of the beaker must also be done with a small folded piece of paper
or paper towel so as to avoid adding weight to the beaker in the form of fingerprints.
You can still weigh your sample directly but do it in a manner which doesn't require as much care
and coordination as that illustrated above by removing the beaker from the pan, adding your
sample and replacing the beaker on the pan. The advantage of this method is that you have more
room for sample addition and the placement of your hands, arms and the transfer spatula is less
awkward, but the disadvantage is that you must check the weight of your beaker and sample after
each addition; it is not good practice to remove excess sample from the beaker if you add too
much. You must be sure that the removed beaker is placed on a clean and dry part of the bench
so to avoid picking up any particles which will change your weight and decrease your accuracy.
The removal and replacement of the beaker must also be done with a small folded piece of paper
or paper towel so as to avoid adding weight to the beaker in the form of fingerprints.
Using the Top Loading
Balance

Several top loading balances, precise to ±0.001 g, are available for your use in the balance
room. Many professors of Quantitative Analysis do not allow them to be placed in the same
room
where the analytical balances are kept because students often use them to save time when they
ought to be using the analytical balances.

First of all, the top loading balances are less precise by a factor of 10 and secondly, air currents
around the pan can reduce that precision by as much as another factor of 3 or 4. But the top
loading balance is the instrument of choice where precision is not of great importance. Here is
one our top loading balances. It can be "tared" by pressing the front bar, as shown at the
right.

In the drawer below the top loading balances you will find weighing paper. This paper must be
used ONLY on the top loading balances, NEVER on the analytical balances, because there is
always the chance that some of the substance being weighed will stick to the weighing paper
after
the weight has been recorded thus producing an error on the low side. The precision of the top
loading balances is ±0.001 and it is possible to see particles which have a mass of 0.001 g.
Moreover, often one wants to weigh a reagent which is to be used in excess, and a precision of
±0.001 is well beyond what is needed for such a weighing.

Once a piece of weighing paper has been placed on the pan, and the balance set to 0.000 by taring
it, a sample can be removed from the container holding it and placed on the pan, repeating the
process as many times as are necessary until the weight of material needed is achieved, as shown
here.








 The tried-and-true method of transferring a precisely weighed sample uses "weighing by
difference," shown here. The empty balance is tared, then the weighing bottle with cap is placed
on the pan and weighed to ±0.0001 g.
The tried-and-true method of transferring a precisely weighed sample uses "weighing by
difference," shown here. The empty balance is tared, then the weighing bottle with cap is placed
on the pan and weighed to ±0.0001 g.  The weighing bottle is removed in a manner which avoids the
transference of oil or other matter from one's fingers.
The weighing bottle is removed in a manner which avoids the
transference of oil or other matter from one's fingers.  The cap is likewise removed from the weighing bottle.
The cap is likewise removed from the weighing bottle. 
 The weighing bottle is tipped above the container to receive the sample and a small amount is
allowed to fall out of the weighing bottle. The weighing bottle is tipped back up and tapped
gently to make sure all of the substance falls back in the bottle and doesn't remain on the bottle
rim. The cap is replaced and the bottle weighed once again. The difference between the first and
second weighings represents the amount transferred. If your
sample has a tendency to absorb water and thus to gain weight when exposed to the moisture of
the air, this method MUST be used to minimize exposure to the atmosphere. Still, the method is
not foolproof and has its own perils: (1) several transfers may be necessary until an amount close
to that needed is
added to the receiving container, (2) too much may be transferred the first time, forcing one to
discard the entire sample ("Drat and blast, I have to start over," is a common epithet heard in
weighing rooms), (3) sample which remains on the weighing bottle rim may be lost and produce
a
weighing error. This peril is repeated each time a transfer is attempted.
The weighing bottle is tipped above the container to receive the sample and a small amount is
allowed to fall out of the weighing bottle. The weighing bottle is tipped back up and tapped
gently to make sure all of the substance falls back in the bottle and doesn't remain on the bottle
rim. The cap is replaced and the bottle weighed once again. The difference between the first and
second weighings represents the amount transferred. If your
sample has a tendency to absorb water and thus to gain weight when exposed to the moisture of
the air, this method MUST be used to minimize exposure to the atmosphere. Still, the method is
not foolproof and has its own perils: (1) several transfers may be necessary until an amount close
to that needed is
added to the receiving container, (2) too much may be transferred the first time, forcing one to
discard the entire sample ("Drat and blast, I have to start over," is a common epithet heard in
weighing rooms), (3) sample which remains on the weighing bottle rim may be lost and produce
a
weighing error. This peril is repeated each time a transfer is attempted.

 The direct transfer of samples to receiving containers is possible using modern analytical
balances
which allow one to tare a receiving container before the transfer is begun. Note the Erlenmeyer
flask on the pan. The Erlenmeyer flask needs to be
completely dry so that there will be no systematic error due to the evaporation of water.
The direct transfer of samples to receiving containers is possible using modern analytical
balances
which allow one to tare a receiving container before the transfer is begun. Note the Erlenmeyer
flask on the pan. The Erlenmeyer flask needs to be
completely dry so that there will be no systematic error due to the evaporation of water. 



 The mass limit of our analytical balances beyond which one receives an error message is
200.0000
grams. Even this 400 mL beaker can be placed on the pan. Note that its mass is just above 149
g.
The mass limit of our analytical balances beyond which one receives an error message is
200.0000
grams. Even this 400 mL beaker can be placed on the pan. Note that its mass is just above 149
g.  Once tared, the sample may be placed directly into the beaker, as shown below.
Once tared, the sample may be placed directly into the beaker, as shown below.


 You can still weigh your sample directly but do it in a manner which doesn't require as much care
and coordination as that illustrated above by removing the beaker from the pan, adding your
sample and replacing the beaker on the pan. The advantage of this method is that you have more
room for sample addition and the placement of your hands, arms and the transfer spatula is less
awkward, but the disadvantage is that you must check the weight of your beaker and sample after
each addition; it is not good practice to remove excess sample from the beaker if you add too
much. You must be sure that the removed beaker is placed on a clean and dry part of the bench
so to avoid picking up any particles which will change your weight and decrease your accuracy.
The removal and replacement of the beaker must also be done with a small folded piece of paper
or paper towel so as to avoid adding weight to the beaker in the form of fingerprints.
You can still weigh your sample directly but do it in a manner which doesn't require as much care
and coordination as that illustrated above by removing the beaker from the pan, adding your
sample and replacing the beaker on the pan. The advantage of this method is that you have more
room for sample addition and the placement of your hands, arms and the transfer spatula is less
awkward, but the disadvantage is that you must check the weight of your beaker and sample after
each addition; it is not good practice to remove excess sample from the beaker if you add too
much. You must be sure that the removed beaker is placed on a clean and dry part of the bench
so to avoid picking up any particles which will change your weight and decrease your accuracy.
The removal and replacement of the beaker must also be done with a small folded piece of paper
or paper towel so as to avoid adding weight to the beaker in the form of fingerprints.




