
The small heating pads available in the laboratory are large enough to hold the three 400 mL beakers to be used in this experiment, as shown in the photo at the right. Heating all three in such close quarters has its dangers, of course, mainly that of tipping one over and losing one sample! So as not to mix up your samples, label each 1,2 and 3 with a graphite pencil.

Notice below how one of the beakers containing the dissolved sulfate sample is placed beneath a buret filled with 5% barium chloride solution. The precipitate is formed by adding the barium chloride slowly to the hot sulfate solution. The slow addition assures that the relative supersaturation always remains low so as to avoid the formation of a non-crystalline colloidal suspension of barium sulfate particles. The aluminum foil seen in this photograph is not necessary as the temperature of the solution is not high enough to melt the paint on the base of the ring stand.

The addition of barium chloride solution can also be done dropwise using an eyedropper
(below right) but you must make sure that the eyedropper is clean before you start, that you do
the addition using a measured amount of barium chloride solution placed in a small beaker and
that you don't allow any barium chloride solution to contaminate the rubber bulb of the
eyedropper. Using this method allows you to add the barium chloride solution to the beakers
while they are continuing to be heated on the hot pads. By way of some final observations, as the filter paper is heated and loses its moisture and
then begins to distill destructively, turning to carbon and releasing the inevitable tars from the
thermal destruction of the cellulose, it is important to cover it so as not to allow it to burst into
flame because the turbulence might allow some barium sulfate caked on it to escape with the
fumes.

The filtration will be carried out using glass or plastic funnels fitted with ashless filter paper.
Ashless filter paper is pure cellulose and will decompose in the presence of heat and air to water
and carbon dioxide. No residual non-volatile substances remain. Ashless filter paper comes in
the
form of circles which must be folded appropriately to trap all of the barium sulfate precipitate.
Click here to see how to fold ashless filter paper.
The funnel rack (below left) is useful to allow you to carry out the filtration
of all samples simultaneously. Make sure that the filter paper you use
is ASHLESS. Erlenmeyer flasks may be used to catch the filtrate. As each
flask fills up, examine the filtrate to make sure that it is free of any
precipitate.
 If the filtrate samples are clear they may be discarded. Presence
of precipitate suggests either a hole in your filter paper or overfilling
a funnel so that precipitate was not caught by the filter. In either case,
ask your instructor about the procedure to be followed.
If the filtrate samples are clear they may be discarded. Presence
of precipitate suggests either a hole in your filter paper or overfilling
a funnel so that precipitate was not caught by the filter. In either case,
ask your instructor about the procedure to be followed.

As is often the case in laboratory chemistry there is more than one correct way to carry out a
procedure and the same can be said for the incorrect execution of a procedure. Here are four
pictures to illustrate the point. The first two illustrate correct ways of pouring your filtrate and
suspended
precipitate into the filter in the funnel. In the first one on the right note that the student is using a
stirring rod to provide a route for the solution into the filter. The lip of the beaker is sufficiently
close to the filter so that the falling solution never speeds up fast enough to form droplets and end
up above the filter paper or, worse, on the lab bench.

The picture at the left shows the pouring operation near the end of the transfer. A stirring rod is
used again but held in place across the top of the beaker for stability. The lip of the beaker is
again close enough to the funnel not to let any of the solution get away. The only criticism that
might be offered here is that the length of the fall of solution is a little too great. So as to
minimize the possibility of loss owing to solution turbulence as it falls, the path of fall ought to
be
shortened.

The next picture on the right shows a student simply pouring his solution out of the beaker.
Immediately after this photo was taken, the falling liquid curled up onto the beaker owing to the
surface tension of water. Some was lost on the funnel above the filter paper and some dribbled
down the side of the beaker. A stirring rod ought to be used to provide a good path for the
solution being transferred.

The photo immediately to the left shows the use of a stirring rod to provide a path, but the
rod is at an angle of inclination too low to provide a good path for the solution. The path ought
to be more vertical or there is a good chance that some solution will be lost by dribbling off the
rod. In addition, the
distance of the lip of the beaker is too far from the funnel and there is every likelihood that at
some point some of the liquid traveling down the rod will separate from it and be lost from the
analysis.


There certainly is no need to beat this subject to death, but I'll lay two more photos on you then
move on to the rest of these helpful hints. Even though it is slightly out of focus, the photo on
the
left shows a nearly vertical stirring rod. This is about as good as it is going to get. The
possibility
that any of the supernatant is going to dribble down the outside of the beaker under the lip is kept
to a minimum by this technique. Please be cognizant that the rod DOES NOT TOUCH the filter
paper. You must avoid that so as not to make holes through which the precipitate might be lost.
The photo on the right illustrates that one ought to try to pour practically all of the supernatant
through the filter paper before starting on the precipitate. That way the filter paper remains
unclogged by precipitate until the very end. The student is in the process of transferring the
precipitate. This can be done by a combination of low-volume washes from your wash bottle and
coaxing the precipitate out with your rubber policement, as shown here.

Finally, the setup on the right shows a filtrate which resulted from leakage either through or
around the filter paper in the small beaker. The appropriate technique would have been for the
student to fold a new piece of filter paper and place it UNDER the first one and continue
filtering,
including a refiltration of the filtrate with the residue followed by washing as described below
and
then ashing of both pieces of filter paper. In fact the student washed the precipitate off the filter
paper back into the suspension yet to be filtered and threw away the old filter paper. Recovery of
the filter paper from the trash can is not recommended because of the possibility it has picked up
other material, so he has the choice of risking a low result if all precipitate was not washed from
it
or starting over. In any case, the filtrate in the larger beaker is appropriately clear, so there is no
apparent leakage of precipitate into it.
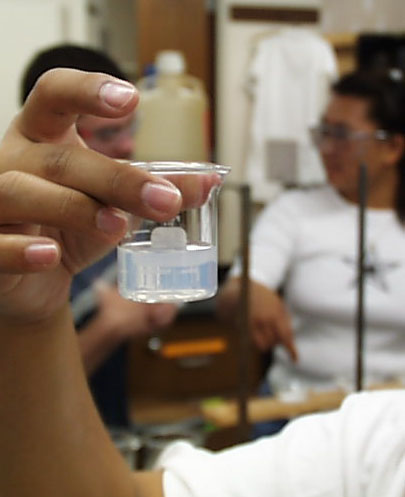
Hot water washes of 15 mL each need to be tested with two drops of silver nitrate solution for
any lingering chloride ion. These three photographs, from left to right show what you might
expect to see as all of the chloride is leached from the precipitate.


Fold your filter paper so that the precipitate is trapped inside and so that the final shape is small
enough to be stuffed into a clean crucible which has been previously weighed. It doesn't matter if
the filter paper is still damp. It may be easier to fold and to stuff if it IS damp, but be careful not
to fold it in a manner which causes the paper itself to rupture because you might lose some of
your precipitate. Note in the image on the left that the filter paper has been stuffed into the
crucible and heating has begun. The image in the middle shows that as the ashing process
progresses
the filter paper blackens and there is the possibility of spontaneous combustion. Keep your
crucible cover ready to extinguish any flame which might produce sufficient turbulence to allow
the escape of fragments of filter paper coated with precipitate. Finally, the image on the right
shows the crucible just prior to final ashing.




 On the other hand, covering the crucible has its perils as well as the photo on the left shows.
Look closely and you can see that the crucible is held in mid-air because the top has stuck to the
rim. The destructively distilled tar can cause the cap to stick to the crucible allowing both to
be lifted together by the cover knob. Don't lift such a pair too high because the cover may
suddenly separate from the crucible causing breakage of the crucible.
On the other hand, covering the crucible has its perils as well as the photo on the left shows.
Look closely and you can see that the crucible is held in mid-air because the top has stuck to the
rim. The destructively distilled tar can cause the cap to stick to the crucible allowing both to
be lifted together by the cover knob. Don't lift such a pair too high because the cover may
suddenly separate from the crucible causing breakage of the crucible.
 Allow the crucible and cover to cool, then gently pry the two apart. The rim of the crucible and
the roof of the cover both will have tar and carbonaceous material caked on them. Note the
underside of the crucible cap in the photo at the left
Allow the crucible and cover to cool, then gently pry the two apart. The rim of the crucible and
the roof of the cover both will have tar and carbonaceous material caked on them. Note the
underside of the crucible cap in the photo at the left

Both can be subjected to heating and ultimately lose all of that residue as shown in the photo
immediately on the right. If there is any suspicion that some of the barium sulfate may have
deposited on the roof of the cover, you may weigh it after heating it thoroughly to remove all of
the carbonaceous material , then wash it, heat it to dryness and weigh it again. Experience has
shown though that rarely does any barium sulfate deposit with the destructively distilled residue
on the cover.

 Even if you have a speck of black material, it is good to continue heating for a while to get rid of
it if it is carbonaceous material as shown here in the photos at the left and the right.
Even if you have a speck of black material, it is good to continue heating for a while to get rid of
it if it is carbonaceous material as shown here in the photos at the left and the right.



 The carbonaceous material on the underside of a cover can be quickly removed by heating it
upside down. The one at the left was heated as shown and the carbonaceous material oxidized
and disappeared in a matter of minutes.
The carbonaceous material on the underside of a cover can be quickly removed by heating it
upside down. The one at the left was heated as shown and the carbonaceous material oxidized
and disappeared in a matter of minutes.