
Heating a Crucible to Constant
Weight
by Oliver Seely
This Web page and accompanying photos are in the public domain and may be copied
without restriction.
Your first exercise teaches you some skills on the proper use of the laboratory burner (in this case called a Tirill Burner), the adjustment of the flame and the proper placement of a crucible which is to be heated to constant weight.

 You ought to make sure at the outset that the crucibles you use have indelible identifying marks
on them. Sometimes there are indentations left during the manufacture of a crucible which will
allow you to distinguish one from another. More likely, there will be a letter or number written
into the side by a former student, such as the one shown at the left. Note the "A" scratched into
the glossy surface. This can be done with the diamond pencil available in the lab, as shown at the
right.
You ought to make sure at the outset that the crucibles you use have indelible identifying marks
on them. Sometimes there are indentations left during the manufacture of a crucible which will
allow you to distinguish one from another. More likely, there will be a letter or number written
into the side by a former student, such as the one shown at the left. Note the "A" scratched into
the glossy surface. This can be done with the diamond pencil available in the lab, as shown at the
right.

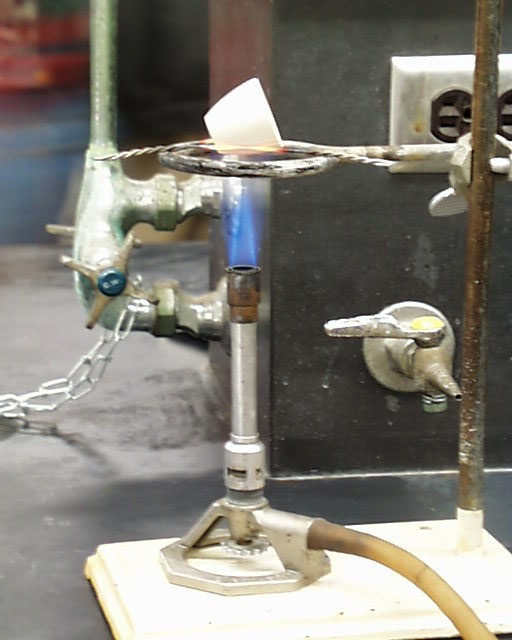
The hottest part of the flame is at the top of the bright cone of oxidation. The zone beneath
the sharp point of the cone is filled with a rising mixture of cool gases not yet in the process of
combustion. Although the rim around this zone is quite hot, the gases inside are essentially at
room temperature. See that placement on the left. Such a
placement is not recommended Note the correct placement of the crucible in the photograph at
the right.


The crucible may be placed right side up, as in the left photo, or on its side, as in the right
photo. Heating it on its side is preferred because the overall strain on the ceramic material at the
high temperatures used is somewhat less.

The crucible ought to be heated to incandescence for 5-10 minutes (left). At the end of this
time, remove the flame and allow the crucible to cool on the iron triangle for a few minutes (right)



When transferring a crucible to the dessicator tongs must be used. If the crucible has been
allowed to cool, you may use bare tongs (left), but if you must transfer a hot
crucible, make sure that you cover the ends of the tongs with aluminum foil (right) as the paint on
the tongs might melt onto the crucible and change its weight. Make sure you grab the crucible on
the edge as shown. Don't try to pick up a crucible by squeezing the tongs on the outer rim both
because the crucible may slip from your grip owing to its slick walls. There is also the possibility
of breaking a hot crucible that way.

Place the cool crucible into one of the holes in the plate in your dessicator and cover until it
has reached room temperature. At that time you may remove it for weighing.
 If a crucible is not at the temperature of the balance compartment it will likely show a weight
different from that it would show were it at the same temperature. If it is hotter the weight will be
slightly less, if colder slightly more owing to the production of convection currents which affect
the apparent mass. Here are some photos to prove the point. (Please note that two separate
analytical balances had to be used for this sequence of photos so as not to interfere with student
use. The final agreement is within that which is routinely observed for two separate balances.) A
dry cold crucible is weighed to the nearest 0.0001 g.
If a crucible is not at the temperature of the balance compartment it will likely show a weight
different from that it would show were it at the same temperature. If it is hotter the weight will be
slightly less, if colder slightly more owing to the production of convection currents which affect
the apparent mass. Here are some photos to prove the point. (Please note that two separate
analytical balances had to be used for this sequence of photos so as not to interfere with student
use. The final agreement is within that which is routinely observed for two separate balances.) A
dry cold crucible is weighed to the nearest 0.0001 g.

The crucible is then heated to incandescence for five minutes, as shown at the left. The fire is
extinguished and the crucible is allowed to cool for five minutes.
 Its apparent weight is less by more than four milligrams.
Its apparent weight is less by more than four milligrams.
It is allowed to cool for an additional five minutes.
 Its exhibited weight is still 0.0003 g less than that of the cold crucible, above.
Its exhibited weight is still 0.0003 g less than that of the cold crucible, above.
 Finally, after an additional five minutes of cooling, the crucible exhibits a weight slightly greater
than that of the cold crucible, above. The agreement here is within that which is routinely
observed for two separate balances. This cooling sequence took place outside the dessicator.
The amount of time necessary to achieve thermal equilibrium with the environment will likely be
somewhat longer when you let the crucible cool in the dessicator.
Finally, after an additional five minutes of cooling, the crucible exhibits a weight slightly greater
than that of the cold crucible, above. The agreement here is within that which is routinely
observed for two separate balances. This cooling sequence took place outside the dessicator.
The amount of time necessary to achieve thermal equilibrium with the environment will likely be
somewhat longer when you let the crucible cool in the dessicator.