 Here is a supersaturated solution of sodium acetate in water..
Here is a supersaturated solution of sodium acetate in water.. Crystallization of Sodium Acetate from a
Supersaturated Solution
by Oliver Seely
This Web page, accompanying photos and video are in the public domain and may be copied
without restriction. All files are located in the same subdirectory in which you find this page.
An aqueous solution can be rendered supersaturated by first dissolving the solute in water at
an elevated temperature using enough to give a concentration just under its solubility at that
temperature. After the last of the solute crystals have dissolved the solution is cooled. The
cooled solution has a concentration above the saturation point and is said to be
supersaturated. Crystals do not form unless the cooled solution is disturbed in
some way, most often by allowing it to come into contact with a small crystalline fragment of the
substance. The model used to describe this phenomenon is that once a template of the crystalline
form of the substance is made available to the supersaturated solution, spontaneous
crystallization
begins immediately.
 Here is a supersaturated solution of sodium acetate in water..
Here is a supersaturated solution of sodium acetate in water..
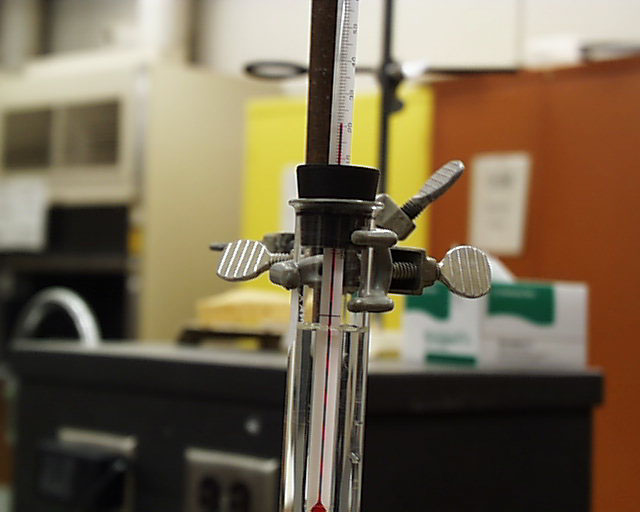 The stopper is removed and replaced with a stopper holding a thermometer. Note that the
temperature is between 20OC and 21OC.
The stopper is removed and replaced with a stopper holding a thermometer. Note that the
temperature is between 20OC and 21OC.
<<<<<<<<<
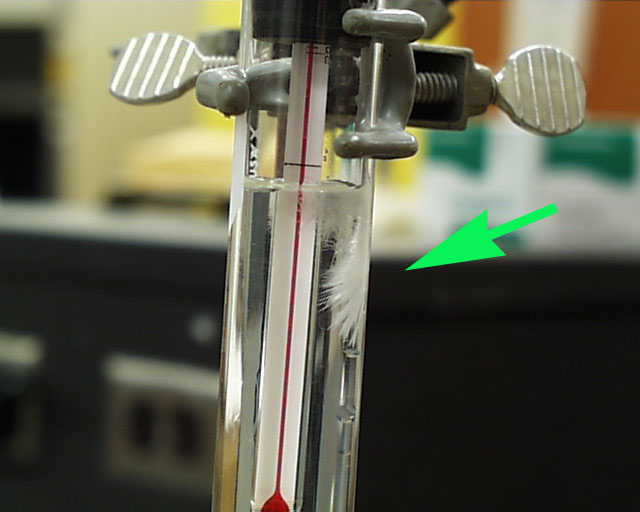 Often, that change in environment is enough to start crystallization, though in this case it did not.
The stopper is loosened and a few seed crystals are introduced on a spatula to the top of the
supersaturated solution. In this solution there is a top layer which is not supersaturated owing to
some distilled water which was introduced to wash down the inner wall just prior to heating.
Note that the crystals begin to grow immediately at the spot on the right of the thermometer
where the seed crystals fell to the point of supersaturation.
>>>>>>>>>
Often, that change in environment is enough to start crystallization, though in this case it did not.
The stopper is loosened and a few seed crystals are introduced on a spatula to the top of the
supersaturated solution. In this solution there is a top layer which is not supersaturated owing to
some distilled water which was introduced to wash down the inner wall just prior to heating.
Note that the crystals begin to grow immediately at the spot on the right of the thermometer
where the seed crystals fell to the point of supersaturation.
>>>>>>>>>
 Within a few seconds, the crystals have grown almost to the bulb of the theromometer.
<<<<<<<<<
Within a few seconds, the crystals have grown almost to the bulb of the theromometer.
<<<<<<<<<
 The crystals grow past the bulb.
>>>>>>>>>
The crystals grow past the bulb.
>>>>>>>>>
 Finally, the crystallization process is complete and has changed the appearance of the contents
from a transparent solution to an inhomogeneous and opaque mixture.
<<<<<<<<
Finally, the crystallization process is complete and has changed the appearance of the contents
from a transparent solution to an inhomogeneous and opaque mixture.
<<<<<<<<
 The final temperature
is more than 47 OC. The starting temperature was just below 21
OC. The rise in temperature during the crystallation demonstrates that the process
of crystallization of sodium acetate is an exothermic process.>>>>>>
The final temperature
is more than 47 OC. The starting temperature was just below 21
OC. The rise in temperature during the crystallation demonstrates that the process
of crystallization of sodium acetate is an exothermic process.>>>>>>
The demonstration below is at the low resolution available to us around 1995 when the video
was made. It was a two-camera setup with the cameras being controlled by the technician in a
control room. Clicking on the link may produce a window which allows you to click on the
name of the file to see the video.
Click here to see the demonstration showing the process and its
exothermic nature.
If you are a PC user and wish to have control over where the downloaded video file is stored,
hold the shift key down as you click the mouse on the link above and then indicate the
subdirectory where the file is to go. Once you know the location of the stored file, you
have the option of choosing either Real Player or Windows Media Player to view the file.
Click here to return to
Oliver's Index of Chemical Demonstrations and Helpful Hints in Laboratory