
The High Vapor Pressure of Diethyl Ether, a
Chemical Demonstration
by Oliver Seely
This Web page, accompanying photos and video are in the public domain and may be copied
without restriction. All files are located in the same subdirectory in which you find this page.
The atoms and molecules which compose a volume of liquid or solid are in constant motion
with a
distribution of energies predicted by the kinetic molecular theory. Depending on the total forces
holding them in proximity to each other and their average kinetic energy (as measured by the
absolute temperature), there will be some tendency for the most energetic molecules to overcome
the forces holding them together and to escape into the gaseous state. Molecules at high
temperature with weak intermolecular forces have a greater tendency to do this than molecules
with strong intermolecular forces at a lower temperature. The resulting collection of molecules in
the gaseous state enter an equilibrium with those remaining in the solid or liquid state. The
gaseous molecules are themselves in constant motion and in colliding with the boundaries of the
container exert a pressure reflective of their numbers and the absolute temperature. This pressure
is called the VAPOR PRESSURE. Owing to differences brought about by intermolecular forces,
each pure substance exhibits a characteristic vapor pressure as a function of temperature.
The table below shows the vapor pressure in torr for water and diethyl ether at six
temperatures:
| Temperature, oC | Water | Diethyl Ether |
| 17 | 14.5 torr | 335.6 |
| 18 | 15.5 | 349.7 |
| 19 | 16.5 | 364.4 |
| 20 | 17.5 | 379.6 |
| 21 | 18.6 | 395.3 |
| 22 | 19.8 | 414.0 |
The volatility of diethyl ether is evident from a vapor pressure more than 20 times that of
water at
these temperatures.


This difference can be demonstrated by means of a buret clamped upside down in a
reservoir of water, several mL of air trapped in the buret above a column of water and a funnel
attached to the buret nozzle:

The buret valve is opened to allow the water to pass almost completely from
the funnel into the buret. Care is taken not to allow air to get trapped in the nozzle of the funnel
leading to the buret.

Note the water flowing down inside on the left inner wall of the buret in the photo on the left.
The trapped bubble of air remains just under the valve thanks to its lower density, but the water is
able to flow around it into the water below.


A small volume of diethyl ether is poured into the funnel and the buret valve is opened to
allow some of the ether to enter the buret column.




For the purpose of more convenient comparisons a second run was carried out in which the
starting meniscus was close to 50mL and the level of the water reservoir at 0 mL:
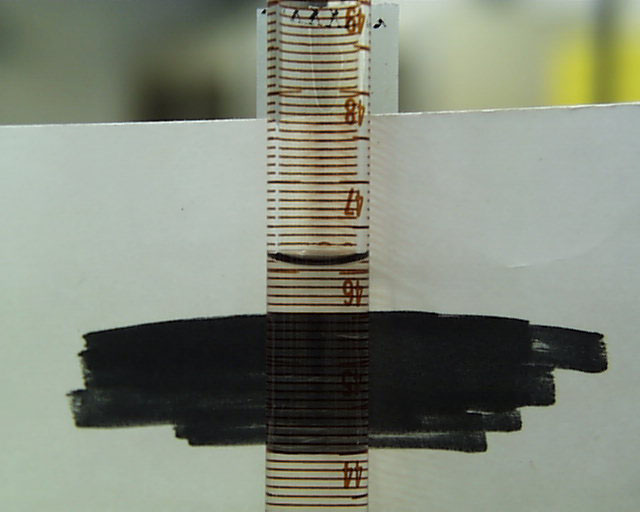
Following the admission of the diethyl ether, the meniscus falls to 46.1 mL, substantially
increasing the volume of the gaseous phase.
Click here to see the
demonstration in Real Video.
The full demonstration ends with a cutoff in mid-sentence, "O.K., now I'm going to bring the
diethyl ether do. . ."
Click here to see the
demonstration in MPEG format
and compatible with Windows Media Player and Real Player Plus G2. (File size =15 MByte)
Downloading this file will take just 4 minutes with a T3 connection and about 90
minutes with a 28.8 kbaud modem.
If you are a PC user and wish to have control over where the downloaded video file is stored,
hold the shift key down as you click the mouse on the link above and then indicate the
subdirectory where the file is to go. Once you know the location of the stored file, you
have the option of choosing either Real Player or Windows Media Player to view the file.
Click here to return to
Oliver's Index of Chemical Demonstrations and Helpful Hints in Laboratory