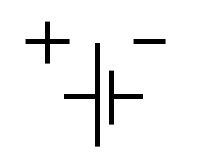
The symbol which describes a cell is shown at the left. The long and short lines represent the two poles or electrodes of the electrochemical cell. Those poles happen also to represent the two different metals. The positive pole is the one where reduction takes place, that is, the location or electrode which "soaks up" electrons or acts as an electron "sink". The electrons from the outside which are absorbed by this metal become available to the cations in solution producing a reduction to the metallic state. The cations are in contact with the electode surface and upon the absorption of enough electrons to become an uncharged metal atom, bonding occurs with the metallic surface and the atom is deposited becoming a part of the electrode. The negative pole or electrode is the one where electrons are being produced thanks to the oxidation of the metal, with the positive ions going into solution and leaving the electrons behind.
The electrical "pressure" or propensity for this two part process to take place is called "chemical potential" or perhaps "electrochemical potential." In honor of the work of Volta, we name that potential after him and call it "voltage" or "volts". Volts are sometimes referred to as electrical potential or electromotive force. A size D,C,B or A, AA, AAA or AAAA "battery" is actually a single cell and its electrical potential is rated at around 1.51 volts.
A curious feature of voltage, at least to this writer, is that it is additive, according to the number of cells which are connected to each other. Volta was the first to notice this with his pile. It is said that a large stack of cells produced enough electromotive force to give him a healthy shock when he grabbed the two wires from either end of the pile with both hands.
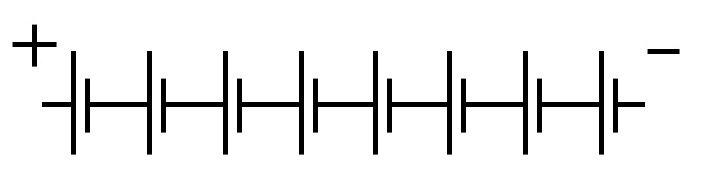
At the right is a symbol for a battery, made up of 8 cells. The voltage of that battery would be 8 x 1.51 or 12.08 volts.

The N21/23 battery is rated at 12 volts. Two of them are shown at the left, one which has been cut open to show the eight cells from which it is constructed.

The image at the right is a close-up of several cells. The three at the bottom have the positive side facing up and the four at the top have been turned over to show the negative side. The visible notches were inadvertently cut into the cells when the battery was cut open.