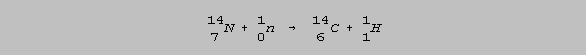
This form of carbon is radioactive. That is, it decays spontaneously to nitrogen 14 by a path involving the emission of a high energy electron (a beta particle):
Oliver Seely
Radiocarbon method
The age of ancient artifacts which contain carbon can be determined by a method known as radiocarbon dating. This method is sometimes called C-14 or carbon-14 dating. Carbon-14 is formed in the upper atmosphere by the bombardment of nitrogen-14 by cosmic rays. Cosmic rays are protons, particles and some heavier ions. Other particles, including neutrons, are produced by subsequent collisions. The collision of a neutron with the nucleus of a N-14 isotope produces C-14, as follows:
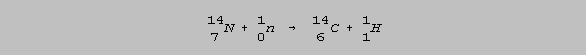
This form of carbon is
radioactive. That is, it decays spontaneously to nitrogen 14 by a path involving
the emission of a high energy electron (a beta particle):
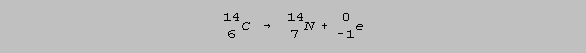
But it decays very
slowly, taking 5730 years for half of a sample of carbon-14 to be converted back
to nitrogen-14.
Samples of wood, charcoal or cloth were originally living vegetable matter. We assume that while living, plants and trees absorb a constant ratio of C-12 and C-14 because the model says that the process of cosmic ray bombardment continues essentially at a constant rate. Since animals are a part of the food chain which includes plants, they also receive a constant ratio of C-12 and C-14, but in the form of carbohydrates, proteins and fats.
The amount of C-14 in any sample of carbon containing material can be found by measuring the level of radioactive decay, and comparing that with the decay rate observed in a carbon sample exposed to the continual mixing at the surface of the earth of C-12 and C-14 produced in the upper atmosphere. Using the ratio of C-14 to total carbon, one can determine the age of the sample.
Since 1/2 of a given sample decays in 5730 years, and half of the remaining sample decays in the next 5730 years, radiocarbon dating cannot be used for samples older than around 60,000 years, or ten half-lives (1/210 = 0.001, or 1/1000 of the original sample). There is evidence gathered from tree rings that the ratio of C-14:C-12 has not remained constant but has varied significantly. Tree ring studies on trees of great ages, such as bristlecone pines and sequoias, provide data to establish a base line ratio of 14C:12C thus increasing the accuracy of the radiocarbon method of dating. The technique was invented by Willard Libby, a professor of chemistry for many years at UCLA. Dr. Libby won the Nobel Prize for his invention of this technique.
A recent celebrated use of radiocarbon dating involved the Shroud of Turin. Some people claimed that the Shroud had been used to wrap the body of the prophet of Christianity after his crucifixion though no one disputed that its history was not known before the 12th century, when it had become the property of the cathedral at Turin, Italy. It was not an official Relic of the Church, but its reputation over the centuries had grown and it probably was responsible for many pilgrimages to the cathedral among the faithful. Early proposals to use radiocarbon dating to determine its age were rejected because such a sizeable amount of material would have to be used to carry out the determination (perhaps as much as 10 cm2 for each sample, and at least 3 samples must be taken to assure reproducibility). The fear was that if its age could be traced to the beginning of the first millennium, then it might well be named a Church Relic -- but one that had to be mutilated to gain that stature. Meanwhile, back at the lab, techniques continued to improve, until reliable radiocarbon dating could finally be done with considerably smaller samples (in the case of the Shroud, just a few short strands were needed for each sample). Such small sample sizes were judged by Church authorities not to constitute mutilation and the analysis went forward. Samples were taken from the Shroud and sent to several laboratories along with other samples of fabrics of known ages. The laboratories were not told which was which. The reported values showed close agreement between the Shroud samples and none suggested an age of the fabric having been harvested from plants before the 12th century A.D. The committee which had taken on the task of judging the validity of the analysis was sufficiently satisfied to convince local Church authorities to retire the claim that it is a Holy Shroud.
Potassium-argon method
There is another often used dating technique for samples considerably older than 60,000 years. It is called potassium-argon dating and is based upon the detected ratio of 40Ar to 40K in a given sample.
Natural potassium is composed of 0.01% radioactive potassium-40 which decays spontaneously according to two routes. 11% converts to calcium-40 by beta decay and 89% converts to inert argon-40 by electron capture (EC). The latter route has a half-life of 1.28 x 109 years:
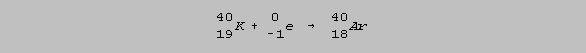
The model says that as molten rock solidifies slowly, dissolved gases are displaced from the
crystalline solid which forms because the gas molecules are excluded from
the crystalline lattice positions. If crystals with uniform lattices form
they may be candidates for potassium-argon dating.
Many minerals contain the element potassium. The radioactive 40K which is
contained in a natural
mixture of potassium isotopes begins to decay to 40Ar gas which gets trapped in the
crystalline matrix.
A sample of ancient rock having an age of billions of years (that is, a piece of rock which was
formed
from molten lava billions of years ago) can be dated using this technique, by grinding the sample
in
a specially built and evacuated container and comparing the ratio of 40Ar to
40K.
Only samples that solidified from the molten state can be analyzed in this manner. Sedimentary rocks which contain potassium cannot be analyzed in this manner because there is no tightly bonded crystal lattice which can trap the gaseous atoms of argon. But sedimentary strata often can be followed to geological faults and other regions where volcanic activity occurred around the same time that the sedimentary rock was deposited. The placement of such volcanic or igneous deposits helps geologists to determine whether the fossil strata are younger or older than the rock which yields to potassium-argon dating methods and such strata can often be dated with underlying and overlying igneous deposits so that one can say with confidence that the strata have an age older than x years but younger than y years.
Interestingly enough, whereas there is an upper limit of around 60,000 years on a sample's age that can be determined using radiocarbon dating, there is a lower limit of around 100,000 years on the age that can be determined using potassium-argon dating. That leaves a gap from 60,000 to 100,000 years that must be filled in with a variety of other dating schemes.